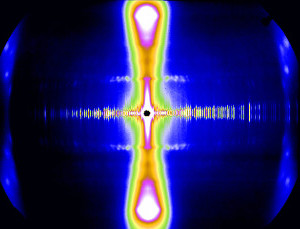
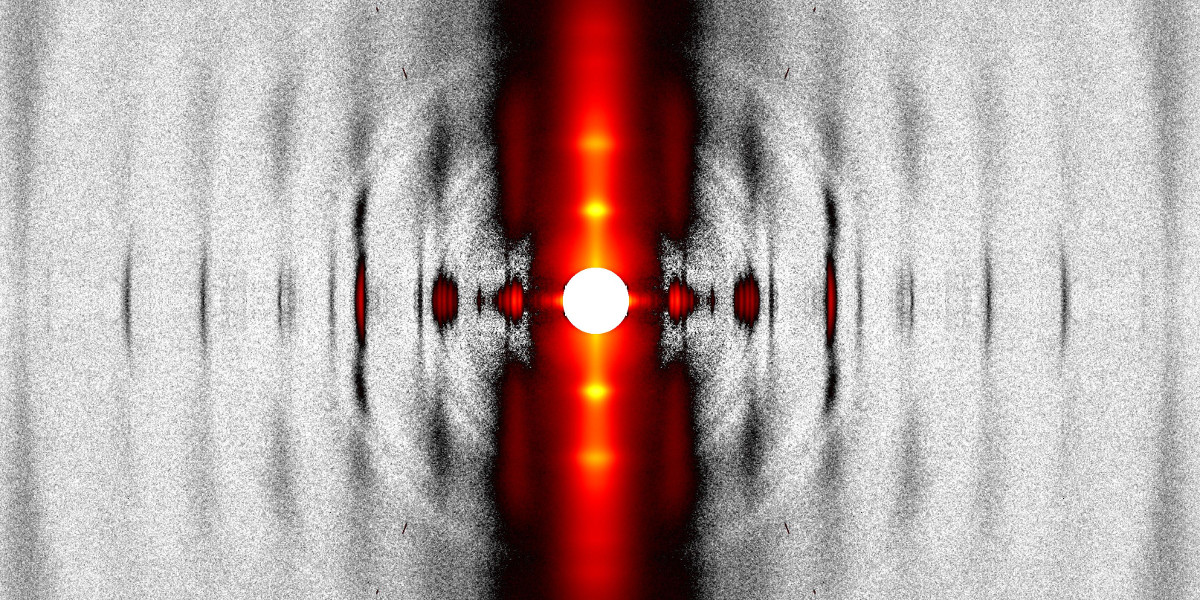
The design features of the BioCAT beamline 18ID and the source properties of the APS allow collection of fiber diffraction patterns of exceptional quality from complex, weakly diffracting biological systems within very short exposure times. The small focal spots achievable with this instrument (~15 x 25 μm2) have allowed excellent discrimination of fine detail in fiber patterns from muscle, connective tissue, and filamentous viruses as well as detection of weak diffraction features in the presence of large backgrounds. The low divergence of the undulator source and the independent horizontal and vertical focusing of our optics simultaneously allows small beam spots at the sample and at the detector that can be varied over a wide range. The high X-ray flux of this instrument (~5.0 x 1013 photons/s) together with the 550 Hz maximum frame rate of the EIGER2 XE 9M detector permits dynamic experiments on these systems with high time-resolution. The large active area of the EIGER2 XE 9M (233 by 245 mm2) allows access to wide q range at high spatial resolution (75 µm).
Facilities for experiments on intact muscle
For combined intact skeletal muscle mechanics and X-ray measurements, we have a vertical muscle mechanics apparatus equipped with one of 3 muscle lever systems (Aurora Scientific 300B series) and a high-power muscle stimulator (Aurora Scientific 701B). The muscle mechanics and X-ray measurements are synchronized by triggering them both from a Dynamic Muscle Control system (Aurora Scientific 605A).
For online intact rat cardiac muscle mechanics and X-ray measurements, we have a horizontal muscle mechanics apparatus equipped with a laser diffraction system including a linear CCD sarcomere length computer system (Dexela Inc., UK), a motor (Aurora Scientific 315C) and a force transducer (Aurora Scientific 400A). The experimental protocol is controlled using a Real-Time Muscle Data Acquisition System (Aurora Scientific model 600). The cardiac muscle fiber is perfused with oxygenated Kreb-Henseleit solution by 2-Channel Minipulse Peristaltic Pump (Gilson Inc). We also have a commercial Langendorf apparatus (Emka Technoligies) that can be used for combined X-ray diffraction/physiological studies of whole, beating rodent hearts under near physiological conditions.
Facilities for experiments on skinned muscle
For online skinned muscle mechanics and X-ray measurements we have a range of horizontal muscle mechanics apparatuses equipped with a range of force transducers (Aurora 400 series), and motors (Aurora Scientific 300 series) for different force levels and response times. He/Ne laser diffraction is available to measure sarcomere length just prior to performing the experiment. We have multiplexed physiology rigs where up to four muscle samples can be mounted at the same time and interrogated sequentially. This also allows for much more efficient experiments, especially when long incubation times are required. The mechanical data can be monitored and recorded by either of our Aurora data acquisition systems described above. These data are also recorded by the main beamline data acquisition system. For remote controlled solution exchange, we have two Hamilton Microlab 500 series dual syringe pumps equipped with multiway valves. Temperature can be adjusted from 10 to 38 °C by a heat exchanger in line with the inlet tubes to the muscle chambers. A PLA based (LulzBot) and a high-resolution resin based (Elego Mars 2) 3D printer on site allow for quick modification and fabrication of experimental chambers and setups based on the users’ needs. We have two fast shutters, each capable of 1 ms exposures, and sub-millisecond exposures can be achieved when both shutters are used in tandem.
Facilities for Fiber Crystallography
Fiber crystallography experiments can use either the standard SAXS instrument using the main beamline optics or the micro-SAXS instrument. The standard SAXS instrument is used when the samples are large and the highest first order resolution required, e.g. experiments where the 67 nm first reflection of collagen needs to be observed. The micro-SAXS instrument is built around a 1.85 m focal length Compound Refractive Lens (CRL) with an angular divergence of 0.4 milliradians in both directions. The delivered flux is 2.2x1012 photons/s at 12 keV into a focal spot of 3 x 3 μm2. When used as a fiber crystallography instrument, the beam focus can be located either at the sample, for the highest spatial resolution in the sample plane or closer to the detector plane for high angular resolution measurements. Our custom modified MAR detector provides a large active area (165 mm image circle) with 40 μm pixels (~60 μm point spread function for the phosphor) for high-resolution data.
The wide-angle fiber crystallography/XFM setup is built on its own optical table at the rear of the experimental enclosure (It is built around a 50 cm focal length CRL with an observed focal spot size of 1 x 0.2 μm2 with a delivered flux of ~ 6 x 1011 photons/s. The short focal length precludes applications where the beam is focused at the detector but provides maximum spatial resolution at the sample position. Diffraction experiments using this device will need to be able to tolerate the relatively high divergence of the beam, ~ 2 milliradians in the detector plane.
Cryogenic sample handling
Capabilities include custom cryoPC cryojet which lacks the drying shield air for large fibrous samples for flash freezing, and 2) a Leica cryo-workstation with liquefied propane for plunge freezing of smaller samples. For the larger tissue samples used in diffraction mapping experiments, we have a custom designed cryo-stage that can maintain larger tissue sections at liquid nitrogen temperature supplied by a liquid nitrogen delivery system (Cryofab model CLBP-25). Any cryogenic experiments need to be done in collaboration with BioCAT staff.
Sample preparation laboratory
BioCAT provides a well-equipped wet lab a few steps away from Beamline 18ID with dedicated space and equipment for sample preparation and characterization. Basic supplies (high-purity water, buffer components, solvents, safety equipment etc.) are provided along with a full suite of 4 °C refrigerators, -20 °C, -80 °C freezers, biohazard storage and disposal. One Zeiss Stemi 2000 and two Zeiss SV6 stereo microscopes are available for dissection and sample characterization. For intact rodent skeletal muscle experiments, there is a bench-top SomnoFlo Low-Flow Electronic Isoflurane Vaporizer from Kent Scientific Corporation that allows obtaining multiple muscle samples form a single animal and/or in situ X-ray diffraction measurements.
There is a recently developed (Spring 2025) staging area (~250 sq ft.) just outside the experimental enclosure for fiber diffraction experiments that allows users to perform final sample manipulations before the X-ray diffraction experiments as well as perform off-line muscle mechanics experiments and biochemical assays. These include a Nikon Eclipse TI2-A inverted microscope with epifluorescence capabilities and digital photo documentation that we have adapted to do mant-ATP pulse-chase experiments for measurements of myosin heads in the so-called Super-Relaxed State (SRX) in parallel with mechanical measurements on myocytes. The staging area also includes facilities for mechanical measurements from myocytes and fibers, a dissection station and a video microscopy station for sarcomere length measurements.
References:
- Books:
- J. M. Squire, The Structural Basis of Muscle Diffraction, Plenum, New York, 1981. (out of print but in many libraries. Good introduction to principles of fiber diffraction)
- Vainstein, X-ray Diffraction from Chain Molecules, Elsevier, 1966.
- C. Cantor and P. Schimmel, Biophysical Chemistry part II: Techniques for the study of Biological Structure and Function Chapter 14. Freeman, 1980.
- Reviews:
- Chandrasekaran, R. and Stubbs, G., “Fibre diffraction,” International Tables for Crystallography, Vol. F: Crystallography of Biological Macromolecules (Rossman, M.G. and Arnold, E., eds.), Kluwer Academic Publishers, The Netherlands, 444-450 (2001).
- Stubbs G., “Developments in fiber diffraction,” Curr. Opin. Struct. Biol., 9, 615-9. (1999)
- Ma, W. and Irving T. Small Angle X-ray Diffraction as a Tool for Structural Characterization of Muscle Disease. Int. J. Mol. Sci. 2022, 23, 3052. https://doi.org/10.3390/ijms23063052