Contents
SAXS sample shreadsheets
BioCAT requests that for each beamtime you send us a spreadsheet of your samples and buffers at least a week before the beamtime starts or you ship your samples (whichever is earlier). Please note that there are two tabs, one for sample and one for buffer. You may also find it useful for tracking/planning your experiments. If you’re not sure what the final concentrations are a week out, send us the spreadsheet with target concentrations, then send us an update once you’ve finalized the preparation. This helps us make sure the appropriate equipment is available for your beamtime, and also lets us recommend any changes that might be needed to sample or buffer volumes/quantities/concentrations ahead of time, ensuring a more successful beamtime.
Checklist for SAXS sample preparation
Below are the recommendations for buffer and sample preparation for SAXS. Note that at the end of the day, these are guidelines and the goal is always the happiness and stability of the sample. We can accommodate most buffers, so ask your beamline scientist if you have an unusual buffer composition.
Also, if possible we recommend combining similar buffers, particularly for separation-coupled measurements like SEC-SAXS. Fewer buffers will save you a significant amount of time during your experiment, so consider whether you can combine similar conditions.
Buffer component recommendations
- 20-100 mM PBS, Tris, HEPES, etc. PBS is preferred if possible.
- Salt concentration < 1M, typically 50-200 mM
- Avoid radical scavengers (glycerol, DTT, etc) unless needed for the stability of your protein. With BioCAT’s coflow setup these actually increase the likelihood of seeing radiation damage.
- If you need a reductant, use longer lived ones like TCEP rather than DTT (especially with higher pH buffers)
Buffer matching
- SEC-SAXS/SEC-MALS-SAXS - Best if buffer is perfectly matched, but can tolerate nominally matched buffers that are not matched through exchange.
- Batch SAXS - Must be a perfect match. Use dialysis buffer, the buffer from the final SEC purification step, or the flow through from the final concentration step.
If mailing concentrated buffer for separation coupled measurements (e.g. a 10x stock for use with SEC-SAXS), we recommend you prepare the concentrated solution, create the final dilution in your lab, exchange your samples into that, and then mail us the remaining concentrated stock for dilution on site. While this doesn’t perfectly match buffer, it gets close, and the buffer exchange on the column will provide the necessary perfect match.
Buffer volume
- SEC-SAXS/SEC-MALS-SAXS - Buffer volume = 4*(column volume)*(number of samples + 1) + 250 mL.
- Batch SAXS - Requires a basic running buffer for the sheath and a perfectly
matched buffer for the sample (can be the sample).
- Sheath buffer: Buffer volume = 0.6*(number of samples) + 50 mL
- Perfectly matched buffer (if not sheath): Buffer volume = 3*(sample volume)
Sample (Protein or DNA/RNA) concentration and volume
- Concentration:
- SEC-MALS-SAXS/SEC-SAXS: 240/(MW in kDa) = concentration mg/ml
- Batch mode SAXS: 60/(MW in kDa) = concentration in mg/ml. Use a dilution series with a least 2 other concentrations bracketed around this. E.g. for a 20 kDa protein, measure at 6, 3, and 1.5 mg/ml.
For nucleic acid samples, you can divide the above concentrations by 2.5.
- Minimum volume:
- SEC-SAXS/SEC-MALS-SAXS - 250 µL
- Batch-mode SAXS - 10 µL per concentration
Note: If mail-in samples are shipped frozen, they will be fast thawed (e.g. in hand/water bath) unless you specify that the samples should be thawed on ice.
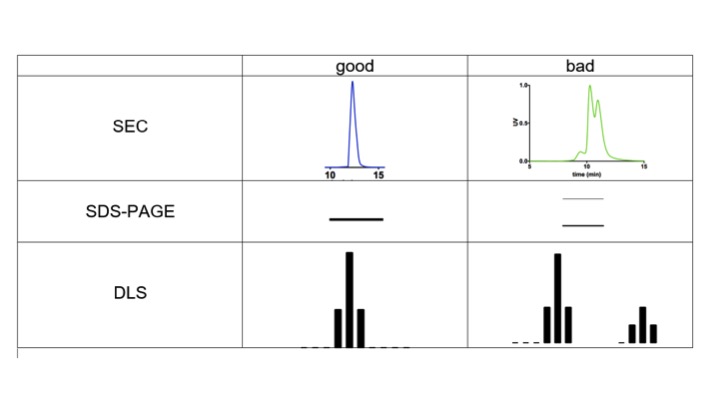
Quality control: Pre-examination before X-ray scattering (Monodispersity)
- Gel filtration (single and symmetric peak)
- SDS-PAGE or Native GEL (single band)
- Centrifugation or filter (> 15,000 x g * 5-10 mins)
- Dynamic light scattering
- SEC-MALS
- Others
More details
In the following video from the 2019 Everything BioSAXS 5 workshop, Dr. Kushol Gupta discusses what you need to know about sample preparation for SAXS (get slides)