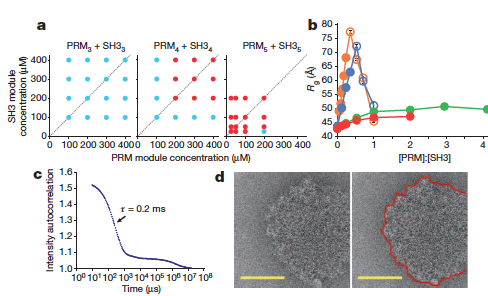
In many biological processes, various substances undergo phase transitions, where they are transformed from one state (solid, liquid, or gas) to another. Wiskott-Aldrich Syndrome Proteins (WASP) function as intracellular signaling molecules, and one member of the family, N-WASP, interacts with two other proteins, forming a complex that plays an integral role in the regulation of the cell’s internal scaffold. This interaction provides a system for investigation of phase transitions that result from multivalent interactions. Using dynamic light scattering (DLS) and small-angle X-ray scattering (SAXS) techniques at the APS, researchers investigated interactions between engineered multivalent substances. They described the occurrence of sharp liquid-liquid-demixing phase separations corresponding to transformation between small complexes and large polymers at the molecular level, leading to the production of micrometer-diameter liquid droplets in aqueous solution. They also examined the phase transition that occurs in relation to N-WASP activity, and found that phosphorylation of one of its interactive proteins by a kinase enzyme plays an integral role in the transformation. Understanding the results of this research will be important in guiding future studies to further evaluate the role of phase transitions in biological systems.
Phase transitions occur widely throughout nature, and involve transformation of a substance from one phase to another, in relation to changes in external conditions. One well-known example is that of evaporation, where liquid water is transformed to water vapor. The interaction of multivalent (multiple unit) small molecules leads to “sol-gel transitions” which involve sharp transformations between small assemblies and large polymer gels. In these processes, the critical transition point at which phase transition occurs relates to the physical properties of the monomeric form of the molecule, and the polymer can take on various physical forms, from liquid to solid.
In biological systems, many processes involve interactions between forms of molecules, including intracellular signaling. The WASP family of proteins acts as intracellular signaling molecules in the regulation of the cellular scaffolding, or cytoskeleton. They are involved in transfer of signals from receptors on the cell surface where they bind, to the inside of the cell where they promote linking together, or polymerization, of filaments of the cytoskeletal protein actin. N-WASP is a member of this family with highest levels in the nervous system, and, together with its 2 protein partners (NCK and phosphorylated nephrin), represents a system for investigation of phase transitions that result from multivalent interactions.
Multivalency has been investigated mostly in relation to extracellular substances binding to receptors on the cell surface. In this context, protein systems aggregate to form cross-linked networks, especially precipitates, but also liquid-like gels. This study, however, aimed to investigate multivalency in relation to intracellular molecules, and in particular to determine whether these systems also experience sharp transformations to polymers.
Using DLS and SAXS techniques at the APS to evaluate reactions, the researchers showed that interactions between synthetic, multivalent substances result in sharp liquid-liquid-demixing phase separations, in which two liquid substances separate to produce 1-50 µm diameter liquid droplets in aqueous solution. At the molecular level, this correlates with a transformation between small complexes and large polymers.
When they investigated N-WASP in this way, the researchers discovered that when it interacts biologically with its 2 protein partners, a phase transition occurs that promotes formation of the Arp2/3 complex - a 7-subunit protein involved in the regulation of the actin cytoskeleton. This complex binds to existing actin filaments, enabling new filaments to grow on the old ones and form a functional actin cytoskeleton. This transformation is regulated by the degree of phosphorylation of nephrin, demonstrating how kinase enzymes can be involved in the control of this aspect of the system.
The exact mechanisms whereby the properties of a substance at the molecular level correlate to those at the macroscopic level are still to be determined. However, this study has provided important insight into the importance of these transformations in biology, providing a mechanism by which multivalent interactions could lead to sharp phase transitions that play an integral role in various important processes, including intracellular signaling. The results of this study will therefore help guide further research in this field, and since multivalent systems are so common throughout nature, it seems possible that phase transitions may be involved in various other aspects of biology such as spatial organization within cells, and biochemical regulation of information transfer.
Adapted from a Advanced Photon Source press release by Nicola Parry
See Pilong Li, Sudeep Banjade, Hui-Chun Cheng, Soyeon Kim, Baoyu Chen, Liang Guo, Marc Llaguno, Javoris V. Hollingsworth, David S. King, Salman F. Banani, Paul S. Russo, Qiu-Xing Jiang, B. Tracy Nixon, Michael K. Rosen, “Phase transitions in the assembly of multivalent signalling proteins,” Nature 483, 336-340 (2012). DOI: 10.1038/nature10879