As humans continue to deplete the Earth’s supply of fossil fuels, finding new sources of energy becomes a priority. Biomass, such as cornhusks left after harvest, is one such alternative energy source. Before efficient use can be made of such materials, understanding how to break down cellulose—the fiber in human nutrition and the main component of much biomass waste—is crucial. With the help of the NE-CAT and BioCAT beamlines at the APS and the SPring-8 (Japan) beamline BL38B1, an international research team from Los Alamos National Laboratory, the University of Tokyo, and the University of Grenoble has identified important new features of cellulose structure. Their work provides important new details that could be used in designing more efficient treatments for cellulosic biomass.
Cellulose is a complicated macromolecule and only a few living things, including the microbes inhabiting the stomachs of cows and other ruminates, have figured out how to metabolize it. Yet biochemists and biophysicists have made significant progress in learning how cellulose is put together and how to break it apart. Among the most promising research in this area is the work done with compounds called amines, which are used in the processing of cellulose and cellulosic biomass. The amines swell the cellulose fibers and make them more susceptible to the cellulase enzymes used in converting biomass to biofuels. The researchers had already studied how amines affect the naturally occurring crystal forms of cellulose, called cellulose I. During the course of their studies, the team found that the amines turned the cellulose into intermediate crystalline aminecellulose I complexes and then, when the amines were removed, into a form called cellulose III that happens to be in an activated state for hydrolysis by cellulases. This latter finding is good news if the goal is to make cellulose easier to turn into biofuels. The researchers were also able to find conditions under which they could turn the cellulose III back into cellulose I, important information for those who want to clearly understand the process and how to control it.
To add further intrigue to the story, cellulose I can also be irreversibly converted to a form called cellulose II, which has improved properties for use in textiles. It turns out that, like cellulose I, cellulose II can also be converted into cellulose III by treatment with amines. As the plot thickened, the research group focused more on visualizing the structure and ways of producing cellulose III, the form that would be most advantageous for biofuels production.
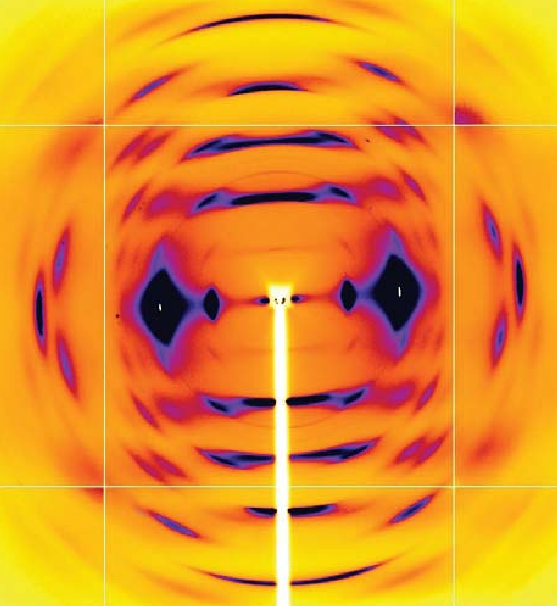
The investigators had previously reported the first high-resolution x-ray data for a crystalline form of cellulose III (IIIII) that they produced by treating cellulose II with ammonia. The team now extended the emerging picture by using crystallography and spectroscopy to determine a new crystal structure for cellulose IIIII. Their data reveal diffuse patterns in cellulose III that indicate a relatively high level of disorder when compared to naturally occurring cellulose chains (Fig. 1). Not only does the cellulose III show more disorder, it also is present in three different types of molecular conformation. The data also led the researchers to propose a new crystal structure for cellulose, a structure that would be consistent with an aggregated microdomain structure for cellulose IIIII. Further work will help determine if modifying the amine treatment of cellulose II will result in a more ordered cellulose III, or whether statistical disorder is an inherent property of cellulose III.
See: Masahisa Wada, Laurent Heux, Yoshiharu Nishiyama, and Paul Langan, “X-ray Crystallographic, Scanning Microprobe X-ray Diffraction, and Cross-Polarized/Magic Angle Spinning 13C NMR Studies of the Structure of Cellulose IIIII” Biomacromolecules 10(2), 302 (2009). DOI: 10.1021/bm8010227
M.W. was supported by a Grant-in-Aid for Scientific Research (18780131). This study was partly funded by the French Agence Nationale de la Recherche. P.L. was supported in part by the Office of Biological and Environmental Research of the Department of Energy, a grant from the National Institute of Medical Sciences of the National Institutes of Health (1R01GM071939-01), and a Laboratory Directed Research and Development grant from Los Alamos National Laboratory (20080001DR). Use of the Advanced Photon Source is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Based on an APS press release by Mona Mort.