Rheumatoid arthritis (RA) is a systemic autoimmune inflammatory and destructive joint disorder that affects tens of millions of people worldwide. Normal healthy joints maintain a balance between the synthesis of extracellular matrix (ECM) molecules and the proteolytic degradation of damaged ones. In the case of RA, this balance is shifted toward matrix destruction due to increased production of cleavage enzymes and the presence of (autoimmune) immunoglobulins resulting from an inflammation induced immune response.
In this study, the authors demonstrate that a polyclonal antibody against the proteoglycan biglycan (BG) causes tissue destruction that may be analogous to that of RA affected tissues. The effect of the antibody is more potent than various harsh chemical and/or enzymatic treatments that have been used to mimic arthritis-like fibril de-polymerization. In the case of RA, the immune response to inflammation causes synovial fibroblasts, monocytes and macrophages to produce cytokines and secrete matrix remodeling enzymes, as well as stimulate B cells to produce antibodies antibodies. The specific antigen that causes the RA immune response has not yet been identified, although possible candidates have been proposed, including collagen types I and II, and proteoglycans (PG’s) such as biglycan. The authors speculate that the initiation of RA associated tissue destruction in vivo may involve a similar non-enzymatic decomposition of collagen fibrils via interactions with antibodies such as they observed here.
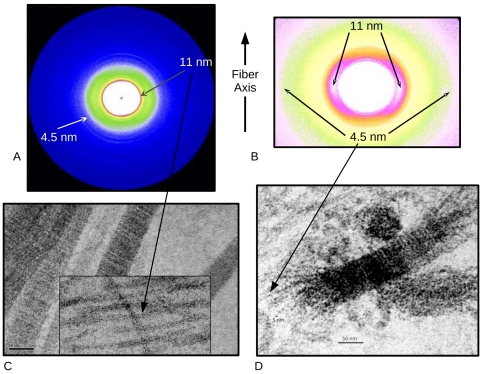
X-ray fiber diffraction at BioCAT was a critical component of the study in that it allowed study of the decomposition of tissues analogous to animal cartilage due to antibodies under unfixed conditions avoiding artifacts that could potentially have been introduced by preparation for electron microscopy. This was of paramount importance to a study that was specifically investigating sample tissue disruption. In addition, XRD was able to identify, for the first time, the existence of type II collagen microfibrils in native tissue, which Antipova and Orgel were then able to confirm in carefully designed TEM preparations.
See: Olga Antipova, Joseph P.R.O. Orgel, “Non-Enzymatic Decomposition of Collagen Fibers by a Biglycan Antibody and a Plausible Mechanism for Rheumatoid Arthritis,” PLoS One 7 (3), e32241-1-e32241-8 (2012).