The stars of muscle contraction—be it the flex of a bicep or the throb of the heart—are microscopic fibers spun from the proteins actin and myosin. To play their parts properly, these proteins need help from an additional, less well-understood molecule known as myosin-binding protein-C. Thanks to data collected at BioCAT beamline 18-ID-D at the APS, researchers from the University of Wisconsin Medical School and the Illinois Institute of Technology have gleaned insights into this protein’s contribution to a smoothly beating heart.
Heart muscle fibers, like those of skeletal muscle, consist of alternating sets of parallel filaments. Thick filaments, which are bundles of myosin molecules, overlap at each end with thin filaments composed of small actin molecules. Connecting the filaments and driving muscle contraction are “cross bridges” that project from myosin. When the muscle is activated, the cross bridges repeatedly grasp, pull, and release adjacent actin, meshing the two sets of fibers together. The effect is akin to a person creeping up a chimney by pushing down with their arms and legs.
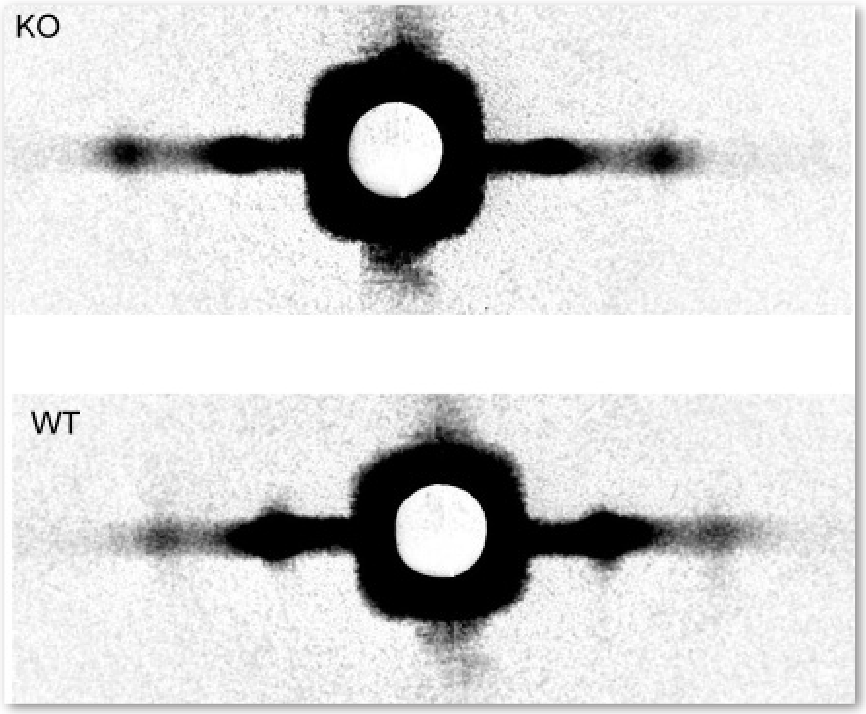
Cardiac myosin-binding protein-C (cMyBP-C) accounts for only 1-2% of heart muscle protein, but it plays a noticeable role. In mice bred to lack the protein, cardiac muscle contracts much more easily than that of normal mice, suggesting that the protein somehow acts as a brake on contraction. To figure out how this works, the researchers first extracted pieces of muscle from inside the hearts of the mice lacking cMyBP-C and soaked the muscle in solution to completely relax it. Next, they probed the muscle’s molecular structure by measuring its x-ray diffraction pattern on beamline 18-ID-D. The diffraction pattern encoded two main properties: the spacing between actin and myosin filaments and the concentration of material along that spacing (Fig. 1). The researchers hypothesized that cMyBP-C works as a tether on cross bridges that happen to be tucked closer than others to their myosin filament at any given moment, preventing them from aiding contraction. In keeping with that idea, they discovered that the molecular mass in the heart muscle without cMyBP-C was less concentrated around myosin. Instead, the molecular mass was approximately 30% more spread out between filaments than in normal mouse heart muscle. The molecular mass was also about 40% more disordered than in normal muscle. The research team interprets the findings as evidence that more cross bridges than normal are projecting farther from the myosin filaments. This would explain earlier physiological studies, because such a change would allow more cross bridges to take part in contraction, resulting in quicker contractions and heightened resting tension in the muscles.
See: Brett A. Colson, Tanya Bekyarova, Daniel P. Fitzsimons, Thomas C. Irving, and Richard L. Moss, “Radial Displacement of Myosin Cross-bridges in Mouse Myocardium due to Ablation of Myosin Binding Protein-C,” J. Mol. Biol. 367, 36 (2007). DOI: 0.1016/j.jmb.2006.12.063
This work was supported by funding from the National Institutes of Health (R3782900) (to R.L.M.). Bio-CAT is a National Institutes of Health-supported Research Center (RR-08630). Use of the APS was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02- 06CH11357.
Based on an APS press release by JR Minkel.