How does one prove that the protein isolated from a 68-million-year-old dinosaur bone is not a contamination from the intervening millenia or from the lab? This is the task of a research team who say they have isolated peptides of the common structural protein, collagen, from bones of Tyrannosaurus rex and Brachylophosauraus canadensis. Although the team had previously presented multiple lines of evidence supporting the veracity of the find, the fact that the age of the peptides far exceeds any previous predictions of how long a protein could resist degradation has generated controversy. In their current work, the researchers used x-ray diffraction data collected utilizing the BioCAT 18-ID x-ray beamline at the U.S. Department of Energy Office of Science’s Advanced Photon Source at Argonne National Laboratory to generate a model of collagen structure on which to overlay the location of the putative dinosaur peptides. The results provide support for a model in which the dinosaur peptides were protected from degradation due to their location within the collagen fibril. This is important evidence supporting the ancient origin of these peptides and the mechanism by which they were preserved. In addition, this new knowledge of collagen structure could be used in the design of highly stable collagenous scaffolds to promote bone and tissue regeneration in humans.
Collagen is a common structural protein found in animals. It makes up about 25% of the human body and is a major component of tendons, ligaments, skin, and bone. Collagen literally holds the body together and its high tensile strength is attributed to its fibrillar structure. Recent evidence has shown that the collagen fibril is made up of microfibrillar units. Three polypeptides wind into a triple helical structure to form a collagen molecule. Five collagen molecules twist around each other to make microfibrils which then pack next to each other to form larger characteristic collagen fibrils. The amino acid sequence of collagen is highly conserved, so it is possible to compare peptides from diverse and ancient species.
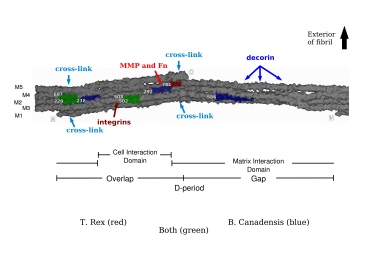
To explain the remarkable durability of dinosaur collagen, the researchers from Orthovita Inc., North Carolina State University, Montana State University, the University of Pennsylvania, the Beth Israel Deaconess Medical Center and Harvard Medical School, the Harvard-Massachusetts Institute of Technology Division of Health Sciences and Technology, The University of Manchester, Manchester, The University of York, York, and the Illinois Institute of Technology (IIT) hypothesized that areas of the protein deeply within the complex fibrillar structure might be preferentially protected from degradation. To test this, they set out to create a model on which to map specific amino acid sequences along and within the collagen fibril to see where their dinosaur peptides matched up. This was achieved by using x-ray diffraction data from the rat tendon collagen I microfibril and fibril in situ collected at the BioCAT beamline to construct a model showing the orientation of the molecules of the triple helix within the microfibril.
According to research team leader Joseph Orgel of IIT, “The approach of our team over the last decade has been to study the structure of collagen in its context, as fibrils located within intact tissue samples. By far our most important work has been in developing the x-ray diffraction techniques and facilities [at BioCAT] to allow us to understand collagen structure in situ. Without this understanding, we would not have been able to perform the analysis undertaken in this recent work.” Using this approach, the team was able identify the location of collagen sequences that are known to interact with other molecules and those which would be expected to be protected in the interior of the fibrillar structure. Sequencing and mapping of 11 dinosaur peptides that represented 8 sequences revealed that the dinosaur sequences were from regions of the protein that were partly protected by molecular packing. This localization could be responsible for protecting the peptides over the millenia.
Further comparison of the sequences to human collagen provided other clues to how these particular peptides might have been preserved. First, there were very few acidic residues found in five of the sequences, meaning their hydrophobic nature would limit their solubility and availability for degradation. Also, few of the peptides represented regions of collagen containing sites targeted by breakdown enzymes and none of them were from the most unstable region of the protein. These features provide hard biochemical evidence for why these particular peptides endured for such a long time.
Does this work satisfy the skeptics? Not yet, but having a new mechanism for how ancient proteins might be preserved is a dinosaur-sized step in the right direction.
Adapted from an APS Scientific highlight by Sandy Field
See: James D. San Antonio, Mary H. Schweitzer, Shane T. Jensen, Raghu Kalluri, Michael Buckley, and Joseph P. R. O. Orgel, “Dinosaur Peptides Suggest Mechanisms of Protein Survival,” PLoS ONE 6(6), e20381 (June 2011). DOI: 10.1371/journal.pone.0020381