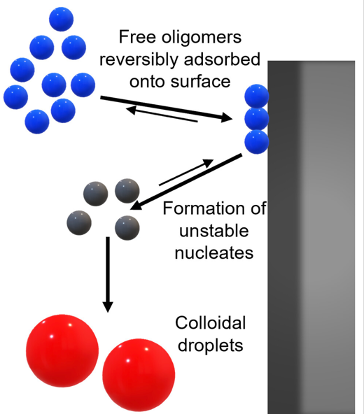
Semaglutide (SMG) is class of modified, acetylated peptide mimic commonly used as a commercial therapeutic to treat type-2 diabetes and obesity. Like other classes of peptide mimic therapeutics, SMG’s suffer from physical instabilities, including various aggregation and degradation pathways but also spontaneous emulsification into colloidal structures in the presence of certain hydrophobic surfaces, a process often termed “ouzo formation.” Researchers at the University of Delaware Center for Neutron Science, in collaboration with Eli Lilly, used a variety of biophysical methods including small-angle X-ray scattering (SAXS), circular dichroism (CD) and dynamic light scattering (DLS) to elucidate the fundamental physical mechanisms behind ouzo formation. SAXS experiments at BioCAT indicated that the colloidal droplets consisted of aggregated oligomers, which themselves form as a result of self-association of the peptides. Together with other experiments, the authors propose a mechanism where initial nucleation is catalyzed by adsorption of oligomers with a hydrophobic surface, where the effects of specific surfaces can be correlated with Hansen Solubility Parameters. Following nucleation (and a subsequent desorption step), nucleated droplets grow rapidly, ultimately resulting in a colloidal dispersion. This work provides a foundation for predicting ouzo-like formation in related molecules, which may help guide future formulations and storage methods for a range of therapeutics.
See: Qi Li, Vasudev Tangry, David P. Allen, Kevin D. Seibert, Ken K. Qian, and Norman J. Wagner. Surface-mediated spontaneous emulsification of the acylated peptide semaglutide. Proc Natl Acad Sci U S A (2024) 121 (5) e2305770121. DOI: 10.1073/pnas.2305770121. PMCID: PMC10835113