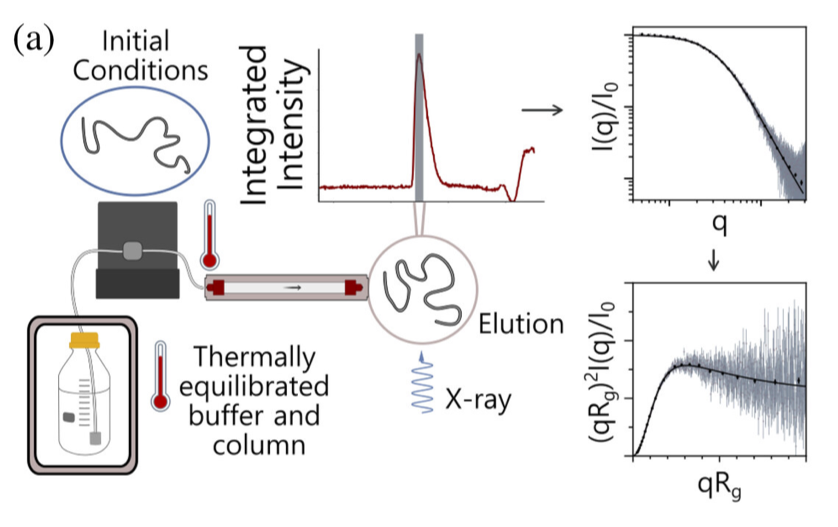
Understanding the driving forces behind stability of denatured state ensembles (DSE’s) and intrinsically disordered proteins (IDP’s) is central to a number of unresolved questions in bimolecular thermodynamics regarding protein folding pathways and foldability, thermodynamic stability, aggregation and misfolding. Researchers at the University of Chicago and Notre Dame used temperature-controlled size-exclusion chromatography-coupled SAXS (SEC-SAXS) and NMR to examine how temperature and solvent ionic strength influences the solution structure(s) of the N-terminal domain of pertactin (PNt). PNt is a valuable model system from a fundamental biophysical point of view, as the full-length 539-residue pertactin folds into a parallel β-helix but the 334 N-terminal residues do not and instead behave as a highly expanded, intrinsically disordered chain.
In this work, the authors used temperature-series SEC-SAXS experiments performed at BioCAT, with temperatures ranging from 4 to 60 degrees C, to show that PNt displays a mild, temperature-dependent contraction (as measured by Rg value). Glycine-rich low hydrophobicity model systems (as proxies for protein backbones alone) did not display an analogous trend, showing the specific importance of side chain interactions. Increasing the ionic strength of the solvent buffer system resulted in an increase in observed PNt chain size over the entire course of the temperature series; at high salt concentrations, PNt collapse appears to be enhanced, likely due to ion exclusion around hydrophobic side chains. The salt and temperature effects appear approximately additive. These results are globally consistent with the known phenomenon of the hydrophobic effect becoming stronger at high temperatures and suggest that the thermodynamic reference state of a DSE may be a function of its environment, resulting in different ΔG values for different modes of denaturation (i.e. thermal vs. chemical). However, extrapolating to a common reference frame still indicates that the various DSE’s (chemical, thermal, native and pressure) are overall thermodynamically equivalent. The authors conclude by postulating that while the hydrophobic effect is the major stabilizing factor in protein folding, additional factors are required for folded stability. This work also raises intriguing questions about how thermophilic organisms have adapted to temperatures above 60 C despite water is generally a poor solvent above those temperatures – in fact, thermophilic proteins often have higher charged residue contents, which this work would suggest further reduces solvent quality for the DSE’s.
See: Michael C. Baxa, Xiaoxuan Lin, Cedrick D. Mukinay,Srinivas Chakravarthy, Joseph R. Sachleben, Sarah Antilla, Nina Hartrampf, Joshua A. Riback, Isabelle A. Gagnon, Bradley L. Pentelute, Patricia L. Clark, Tobin R. Sosnick. How hydrophobicity, side chains, and salt affect the dimensions of disordered proteins. Protein Science. 2024;33:e4986. DOI: 10.1002/pro.4986 PMCID: PMC11010952