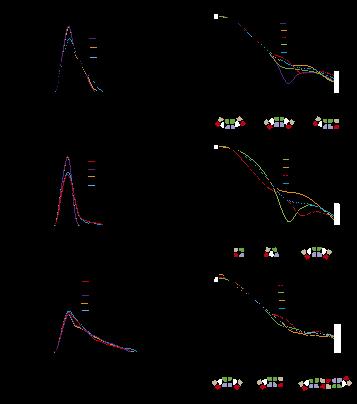
SAXS analysis of IDE. Pair distribution functions and scattering curves of WT IDE and IDE R767A (C and D), and IDE S132C/E817C (E and F). Curve fitting is based on atomic models using the program CRYSOL (single model) or OLIGOMER (mixture). D2/D3, D2/D3 pivot; D1/D4, D1/D4 pivot; C, closed state; M, monomer; D, dimer; T, tetramer. The diagrams and ratios shown below the scattering profiles represent the distribution of mixture that could best fit the SAXS data.
Proteins in living organisms face acute and chronic challenges to their integrity, which require proteostatic processes to protect their functions. Proper protein function is ensured through protein turnover through a balance between synthesis and proteolysis. Amyloidogenic peptides, such as amyloid β (Aβ) and amylin, present a major challenge to proteostasis, because they can form toxic aggregates that impair diverse physiological functions and contribute to human diseases. Insulin-degrading enzyme (IDE), a Zn2+-metalloprotease, prefers to degrade amyloidogenic peptides to prevent the formation of amyloid fibrils. Thus, IDE retards the progression of Alzheimer’s disease.
IDE possesses an enclosed catalytic chamber that engulfs and degrades its peptide substrates; however, the molecular mechanism of IDE function, including substrate access to the chamber and recognition, remains elusive. Here, we captured a unique IDE con- formation by using a synthetic antibody fragment as a crystallization chaperone. An unexpected displacement of a door subdomain creates a ∼18-Å opening to the chamber. This “swinging door” motion uncovered by this study significantly advances our mechanistic understanding of IDE function. The swinging-door permits the entry of short peptides into the catalytic chamber and disrupts the catalytic site within IDE door subdomain. Given the propensity of amyloidogenic peptides to convert into β-strands for their polymerization into amyloid fibrils, they also use such β-strands to stabilize the disrupted catalytic site resided at IDE door subdomain for their degradation by IDE. Thus, action of the swinging door allows IDE to recognize amyloidogenicity by substrate-induced stabilization of the IDE catalytic cleft. Small angle X-ray scattering (SAXS) analysis revealed that IDE exists as a mixture of closed and open states. These open states, which are distinct from the swinging door state, permit entry of larger substrates (e.g., Aβ, insulin) to the chamber and are preferred in solution. Because IDE is a key protease in the destruction of amyloidogenic peptides and novel biologically relevant substrates of IDE, such as MIP-1α and calcitonin gene- related peptide, continue to be discovered (11, 32), future studies will address how conformational dynamics are linked to the catalytic cycle of IDE and how to control such processes. Such information can also provide avenues to design IDE-based therapies for modulating proteostasis in humans.
Citation: Lauren A. McCord Wenguang, G. Liang, Evan Dowdel, Vasilios Kalas, Robert J. Hoey, Akiko Koide, Shohei Koide, and Wei-Jen Tang, Conformational states and recognition of amyloidogenic peptides of human insulin-degrading enzyme PNAS 110 (34): 13827–13832 (2013) PMCID: PMC3752249
This is a classic example of where SAXS can be used to test mechanistic hypotheses regarding large-scale domain movements in proteins. It is also an example where having successful and productive Driving Biomedical Projects (in this case with Prof. Tobin Sosnick at Chicago) at a particular institution can be used to recruit other excellent service projects.