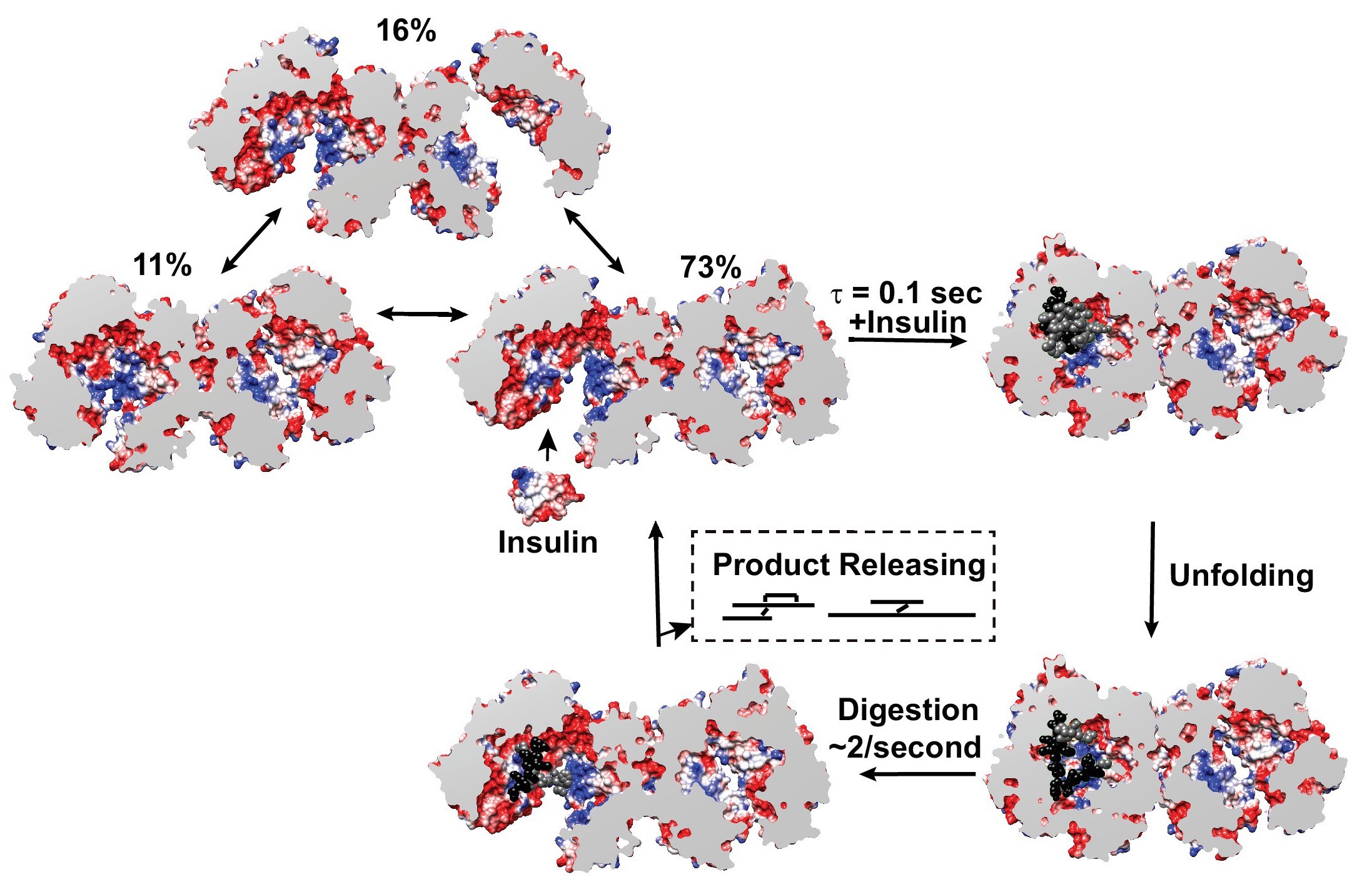
The Tang lab (University of Chicago, Illinois) has a deep interest in the structure and function of Insulin Degrading Enzyme (IDE), a zinc metalloprotease with a role in insulin and amyloid β degradation and known to be a significant factor in the pathophysiology of conditions such as Diabetes mellitus and Alzheimer’s disease. Previous structural studies showed that IDE forms dimers that can assume two distinct and functionally significant conformations. Substrate recognition occurs in an enclosed catalytic site formed by the dimerized IDE mostly via electrostatic interactions and size and shape complementarity. Catalytically important open conformations have been difficult to study using crystallography. The Tang lab used a combination of cryoEM, X-ray crystallography, Hydrogen-Deuterium exchange coupled Mass Spectrometry (HDX-MS), and SAXS to elucidate key mechanistic details. Four heretofore unknown and distinct conformations were determined using cryoEM strongly suggesting that substrate recognition and capture are contingent on the openness of IDE conformation.
Equilibrium SAXS showed that while IDE dimers exist in equilibrium between open and closed conformations, the presence of substrate conformationally restricts IDE to the partially closed or closed state. The question therefore was whether the enzymatic rate of ~ 2 per second determined by enzyme kinetic analysis was attributable to the rate of conformational opening and closing of IDE. BioCAT has been a pioneer in the development of microfluidic mixers usable for time-resolved SAXS measurements. One such development is the laminar flow mixer which provides access to time-regimes in the millisecond to several seconds. The change in conformation from open to closed is represented by a significant difference in the radius of gyration (Rg)(Fig.8). Fast mixing with insulin and monitoring the change in Rg showed that the rate of transition from high to low Rg was compatible with the aforementioned catalytic rate of ~ 2 per second (Fig.9). This project is an illustration of SAXS’s place in scenarios where a multidisciplinary approach is mandated by the complexity of questions that cannot be conclusively answered using any one technique.
See: Zhang Z, Liang WG, Bailey LJ, Tan YZ, Wei H, Wang A, Farcasanu M, Woods VA, McCord LA, Lee D, Shang W, Deprez-Poulain R, Deprez B, Liu DR, Koide A, Koide S, Kossiakoff AA, Li S, Carragher B, Potter CS, Tang WJ. “Ensemble cryoEM elucidates the mechanism of insulin capture and degradation by human insulin degrading enzyme,” eLife 2018, 7:e33572 PMCID: PMC5910022, DOI: 10.7554/eLife.33572.