
A drug normally used to treat Alzheimer’s disease may act as a “copper bullet,” killing tumor cells by coating itself in copper ions, according to research derived in part from studies at the APS. Researchers from Wayne State University, the Henry Ford Hospital, the Illinois Institute of Technology, and Shandong University using BioCAT beamline 18-ID-D at the APS found that the drug clioquinol, when mixed with copper (Fig. 1), killed two types of prostate cancer cell in Petri dishes. The drug without copper also slowed the growth of prostate tumors implanted in mice by up to two-thirds, apparently by soaking up copper ions (charged atoms) present in the implanted tumor cells.
Prostate cancer struck approximately 219,000 U.S. men in 2007 and killed some 27,000 of them, according to National Cancer Institute estimates.
Many types of cancer typically show high levels of copper, including tumors of the prostate, breast, colon, lung, and brain. Traditional chemotherapy works by poisoning any rapidly growing cell, which includes healthy gut, blood, and hair follicles, resulting in hair loss, nausea, and other unpleasant side effects.
If only high concentrations of copper trigger clioquinol’s harmful effects, these researchers noted, then the drug might selectively kill tumor cells, causing fewer side effects than other chemo-therapies. The researchers further noted that two clinical trials of the molecule as a treatment for Alzheimer’s disease did not reveal any significant side effects.
Intrigued by a study reporting that clioquinol shrank lymphoma tumors in rodents, the researchers suspected that copper could be the key, on the basis of previous work in which they observed that copper-containing compounds inhibited a protein “garbage disposal,” called the proteasome, that keeps all cells running properly.
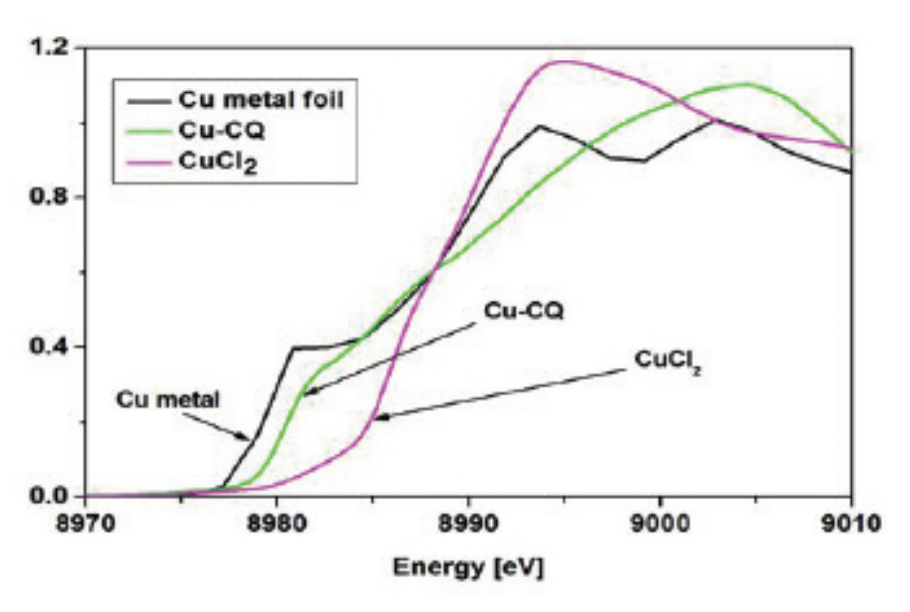
The first task was to confirm that clioquinol reacts with copper and forms a new complex. This was accomplished by x-ray absorption studies performed on the BioCAT beamline (Fig. 2). Compounds in hand, the group discovered that in two human prostate cell lines exposed to the clioquinolcopper “bullets,” proteasome activity fell by up to 69%, which was followed by a loss of cell surface proteins that recognized the male hormone androgen, a known stimulator of prostate tumors. In effect, the molecule seemed to rob the deadly cells of a key signal that told them to go forth and multiply, all by way of the proteasome. As a result, the cells then began to self-destruct.
The compound by itself had similar effects on copperrich human prostate cancer cells growing either in a Petri dish or in rodents. Researchers believe that copper ions promote the growth of new blood vessels that feed tumors, and the team observed that this growth (called angiogenesis) was reduced in the transplanted tumors exposed to clioquinol compared with untreated tumor transplants, suggesting that the molecule inhibited angiogenesis as well as the proteasome by soaking up the available copper.
See: Di Chen, Qiuzhi Cindy Cui, Huanjie Yang, Raul A. Barrea, Fazlul H. Sarkar, Shijie Sheng, Bing Yan, G. Prem Veer Reddy, and Q. Ping Dou, “Clioquinol, a Therapeutic Agent for Alzheimer’s Disease, Has Proteasome-Inhibitory, Androgen Receptor-Suppressing, Apoptosis-Inducing, and Antitumor Activities in Human Prostate Cancer Cells and Xenografts,” Cancer Res. 67(4), 1636 (February 15, 2007). DOI: 10.1158/0008-5472.CAN-06-354
This work was supported by Karmanos Cancer Institute of Wayne State University, Deparment of Defense Breast Cancer Research Program awards W8IX-04-1-0688 and DAMI7-03-I-0175, and National Cancer Institute grant CA112625 (Q.P. Dou); and by the National Cancer Institute/NIH Cancer Center Support grant (Karmanos Cancer Institute). The Biophysics Collaborative Access Team is an NIH-supported research center, RR08630. Use of the APS was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DEAC02-06CH11357.
Based on an APS press release by JR Minkel.