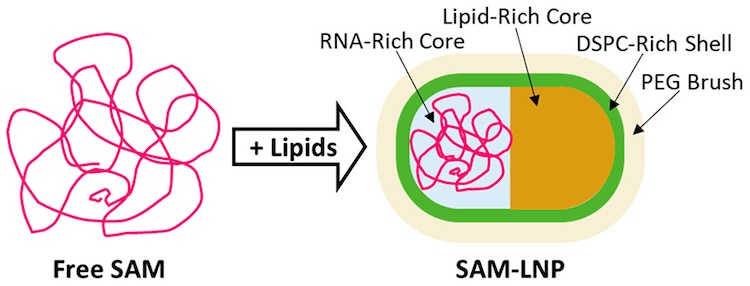
The success of mRNA-based COVID-19 vaccines has brought increased attention to lipid nanoparticles (LNPs) as an RNA therapeutic delivery method. One of the main challenges of LNPs as a delivery method remains the significant percentage of unloaded LNPs in most (if not all) RNA-LNP formulations, highlighting a need for better characterization of their formation, composition and morphology. While there have been a number of recent advances in understanding the formation and structure of LNPs, studies exploring the influence of non-mRNA RNAs on LNP morphology remain limited. Of particular interest is the delivery of self-amplifying mRNA (SAM), which has the potential to both significantly lower dosage as well extend the antigen expression lifetime in the body. However, given that SAM molecules are typically significantly larger than conventional nonreplicating mRNA, and that larger nucleic acids are known to promote the formation of significant populations of empty LNPs, further work is needed to better understand the formation, composition and morphology of LNPs in order to better inform LNPs as a delivery system for promising therapeutic strategies that rely on larger nucleic acids.
Researchers from ORNL, NIST and the GSK Center for Vaccine research characterized the morphology of LNPs encapsulating SAMs using a variety of techniques, including cryo-electron microscopy, contrast-varying small-angle neutron scattering (CV-SANS) and small-angle X-ray scattering (SAXS). Using SAXS data collected at BioCAT, the authors studied the global structure of SAM before and after LNP encapsulation. The pair density distribution functions [P(r)] clearly show that the SAM LNP systems were significantly smaller than free SAM, suggesting that the SAM must compact in order to be loaded into the LNP. Additional cryo-EM experiments showed that SAM LNPs adopt a wide range of sizes and morphologies, including nonspherical and two-compartment morphologies. Finally, by formulating SAM LNPs with distinct isotopic compositions, the authors were able to use CV-SANS to assess the distribution of helper lipids and apply scattering analysis to the SAXS and SANS data to determine that the primary representative morphology of SAM LNPs is in fact nonspherical and asymmetric in its component distribution.
The prevalence of nonspherical morphologies in SAM LNPs is distinct from the more spherical, uniform morphologies seen in siRNA LNPs and is instead more similar to those seen with mRNA. Analysis of their composition indicated that DSPC and PEGylated lipids are common in the outer shell, potentially providing a starting point for varying the particle size and distribution by changing the composition of those helper lipids. This work presents the first model of an RNA-LNP with a nonspherical shape, highlighting the need to explore further the morphological characteristics of RNA-LNPs. In addition to its clear practical applications in medicine, this work is also an excellent example of the strengths of combining SAXS and SANS data, as the techniques excel at highlighting different components of the SAM LNPs. Nucleic acids are highly visible in SAXS due to the high contrast27, while lipids have notably better contrast in SANS. Similarly to their use as validation of high-resolution macromolecular data, both techniques also provide important validation for the morphologies observed in the cryo-EM experiments. Given how rapidly the field is evolving, we expect significant continued interest in scattering studies of LNP formation, composition and morphologies going forward.
This experiment took particular advantage of the extremely low minimum q routinely offered at BioCAT. In order to measure the large maximum dimension of these objects a minimum q of less than 0.0032 Å-1 was required, which is not routinely available at any other U.S. bioSAXS beamline. The growing interest by users in using SAXS to characterize LNPs motived BioCAT to acquire our flow field fractionation instrument to allow in-line size separated measurements on these systems.
See: Thelen, J. L. et al. Morphological Characterization of Self-Amplifying mRNA Lipid Nanoparticles. ACS Nano 18, 1464–1476 (2024)