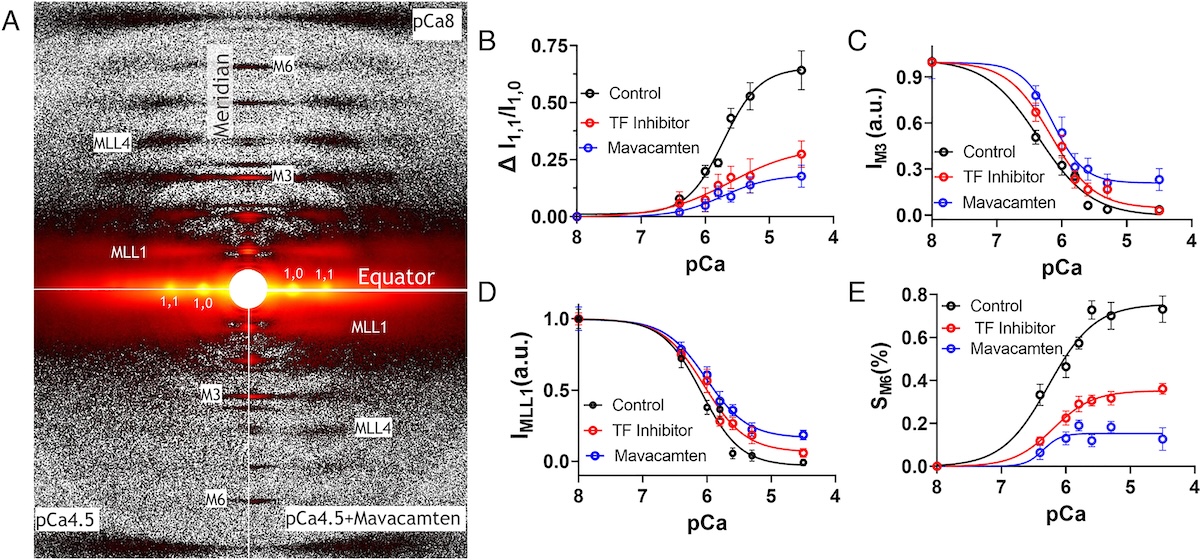
Mavacamten is the first myosin-targeted small-molecule therapy approved by the Food and Drug Administration to treat obstructive hypertrophic cardiomyopathy by attenuating excessive myocardial sarcomere activity. Mavacamten regulates cardiac function at the sarcomere level by selectively but reversibly inhibiting the enzymatic activity of myosin. It shifts myosin toward ordered off states close to the thick filament backbone making them unavailable for binding to actin and generating force. It is necessary, however for the heart to adjust its output to ensure sufficient cardiac output, especially during increased physiological demands. It was unknown whether mavacamten stabilized heads could still be recruited by the usual physiological inotropic mechanisms for the patient to be able to adapt to changing demands on their hearts.
The authors of this study provided direct evidence that all major inotropic interventions are preserved in the setting of mavacamten. In particular, cardiac myosins stabilized in off state(s) by mavacamten were shown to be recruitable by 1) Ca2+, 2) increased chronotropy [heart rate (HR)], 3) stretch, and 4) β-adrenergic (β-AR) stimulation. At the molecular level, X-ray diffraction experiments a t BioCAT showed that Ca2+ was able to increase myosin ATPase activity by shifting mavacamten-stabilized myosin heads from the inactive super-relaxed state to the active disordered relaxed state. At the myofilament level, both Ca2+ and passive lengthening shifted mavacamten-ordered off myosin heads from positions close to the thick filament backbone to disordered on states closer to the thin filaments. In isolated rat cardiomyocytes, increased stimulation rates enhanced shortening fraction in mavacamten-treated cells. This observation was confirmed in vivo in telemetered rats, where left-ventricular dP/dtmax, an index of inotropy, increased with HR in mavacamten-treated animals. Finally, they were able to show that β-AR stimulation in vivo increases left-ventricular function and stroke volume in the setting of mavacamten. Taken together, their data demonstrated that the mavacamten-promoted off states of myosin in the thick filament are at least partially activable, thus preserving cardiac reserve mechanisms.
See: Weikang Ma, Carlos L. del Rio, Lin Qi, Momcilo Prodanovic, Srboljub Mijailovich, Christopher Zambataro, Henry Gong, Rafael Shimkunas, Sampath Gollapudi, Suman Nag, and Thomas C. Irving. Myosin in autoinhibited off state(s), stabilized by mavacamten, can be recruited via inotropic effectors. Proc Natl Acad Sci U S A. 2024 Feb 20;121(8):e2314914121. DOI: 10.1073/pnas.2314914121. PMCID: PMC10895252.