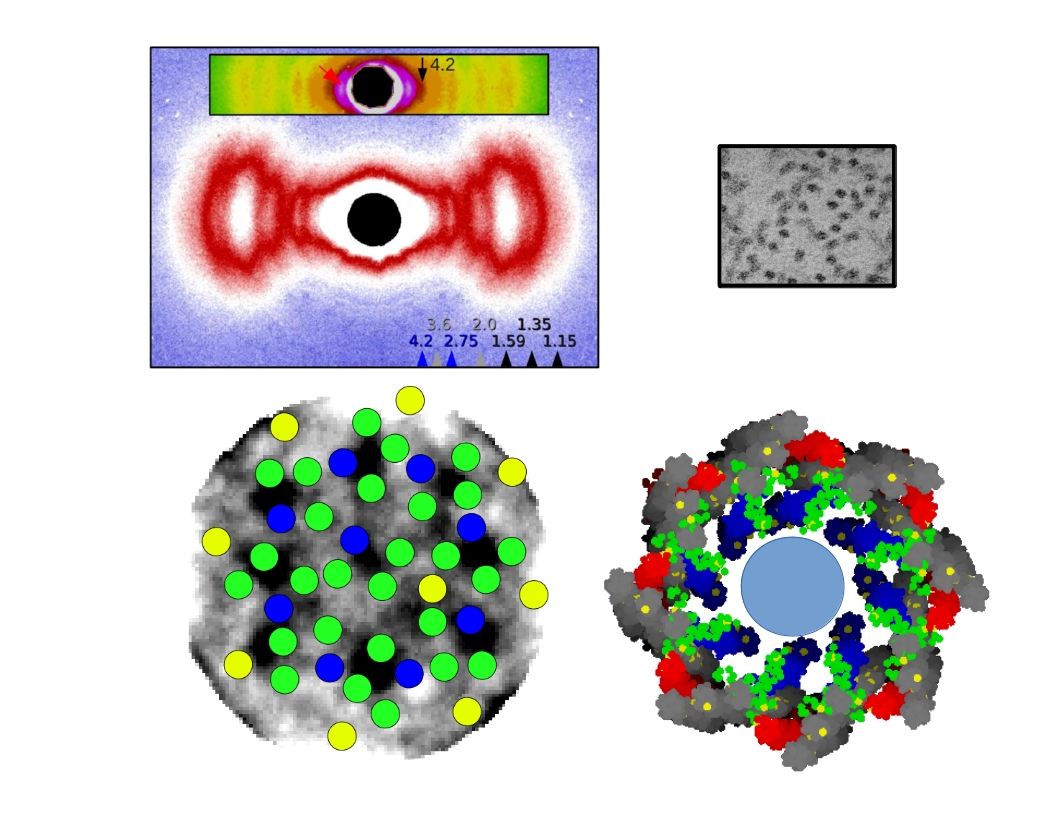
Type II collagen is the principal extracellular matrix (ECM) component of mammalian cartilage. It has been shown that lamprey (a cartilagenus fish) notochord collagen fibrils are indistinguishable from human cartilage fibrils, their differences being minor amino acid sequences and in the specific arrangement of the fibrils to build their respective ECM’s. Recently, the Orgel group demonstrated that a human anti-biglycan antibody had the capacity to ‘deconstruct’ lamprey notochord type II collagen fibril bundles to give a pure source of ‘thin-fibril’ collagen fibrils that are primarily composed of type II collagen. This procedure provides both a possible explanation for the initiation of Rheumatoid arthritis and a means of studying the structural arrangement of tissue.
This work comprised two principal methods: 1) Using highly focused microbeam at BioCAT to locate the most crystalline portions of a notochord sample, and collecting data from this region over extended periods of time (>15 minutes) using a cryojet. 2) Analysis of TEM data from fibril cross-sections to identity the arrangement of collagen microfibrils within the thin-fibrils. Using the first method, the Orgel group determined the quasi-hexagonal packing of the notchord tissue (previously thought to be tetragonal due to limitations in data available) and the 2D unit cell. Using this information and the diameter determined from X-ray diffraction at BioCAT and TEM data a model of the fibril structure was composed. The TEM data of fibril cross-sections was parsed with a custom algorithm and it was found that the microfibrillar arrangement determined in both methods 1 and 2 was the same, a 8+1 collagen fibril arrangement. Making sense for the first time, that the ratio of collagen type II and XI in cartilaginous tissue is 8:1. A pdb coordinate model was composed on this bases and the work will be submitted for publication shortly.
See: Olga Antipova and Joseph Orgel 2012, Nonenzymatic decomposition of collagen fibers by a biglycan antibody and a plausible mechanism for rheumatoid arthritis. PLoS One. 2012;7(3):e32241PMCID: PMC3302792.
The Orgel group has been a collaborator with the BioCAT beamline since shortly after it came on line. The group has worked extensively with small and wide angle diffraction, being an early adopter of micro and cryo diffraction for fibrious substances. Orgel is now Associate Director responsible for scientific leadership in fiber diffraction and closely related scientific areas. This work depended critically on the development of microdiffraction and cryogenic handling techniques for fibrous specimens.