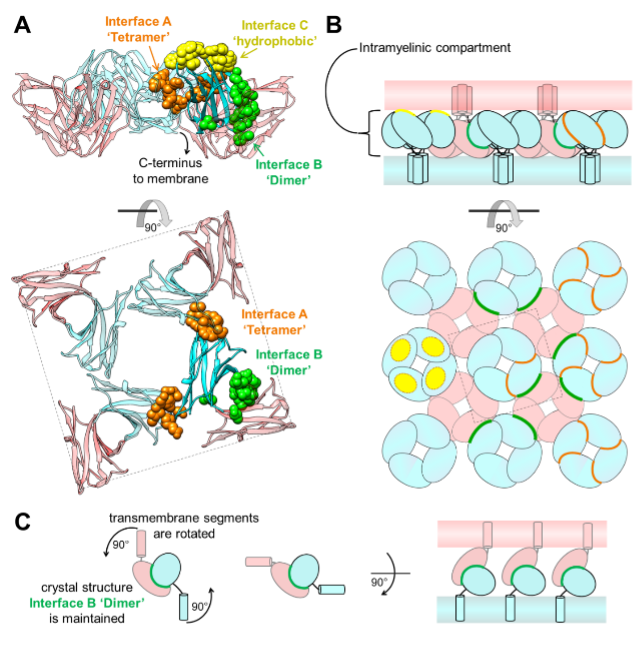
Charcot Marie Tooth (CMT) disease is the most common form of heritable peripheral neuropathy, which are a group of inherited diseases affecting the peripheral nervous system (PNS). Myelin protein zero (MPZ) is necessary for normal myelin structural and function comprises ~50% of all proteins in the PNS; mutations in MPZ account for around 5% of CMT cases. MPZ is a transmembrane adhesion protein which holds together adjacent myelin membranes, thought to be mediated in part through homotypic interactions of its extracellular Ig domain. Exactly how the Ig domain of MPZ (IgMPZ) mediates adhesion of apposing membranes is not yet fully understood but is nonetheless important for understanding how myelin is constructed and how mutations in the IgMPZ cause disease. Models for how the IgMPZ might form oligomeric assemblies has been extrapolated from a protein crystal structure in which individual rat IgMPZ subunits packed together to form 3 weak interfaces involving IgMPZ organized tetramers, a ‘dimer’ interface linking tetramers together, and a hydrophobic interface that mediates binding to lipid bilayers or the same hydrophobic surface on another IgMPZ domain. It was not clear whether the proposed IgMPZ interfaces actually mediate oligomerization in solution, whether they are involved in the adhesion activity of MPZ, are important for myelination, or whether their loss results in disease. The authors performed nuclear magnetic resonance spectroscopy and small angle X-ray scattering (SAXS) analysis of wild-type IgMPZ as well as mutant forms with amino-acid substitutions designed to interrupt presumptive oligomerization interfaces. They confirmed the interface that mediates IgMPZ tetramerization, but found that dimerization is mediated by a distinct interface that has yet to be identified. They were able to correlate different types of Charcot-Marie-Tooth disease symptoms to subregions within IgMPZ tetramers. Using this information, they were able to use computational docking studies to predict how disease-relevant subregions may mediate dimerization of IgMPZ tetramers.
See: Christopher P. Ptak, Tabitha A. Peterson, Jesse B. Hopkins, Christopher A. Ahern, Michael E. Shy, Robert C. Piper. Homomeric interactions of the MPZ Ig domain and their relation to Charcot-Marie-Tooth disease. Brain, (2023). DOI: 10.1093/brain/awad258 PMCID: PMC10690024