It has been known that muscle fibers shorten slowly under heavier loads and faster under lighter loads. Now, researchers from the Università degli Studi di Firenze, Università di Roma, Dexela Ltd., the Illinois Institute of Technology, and King’s College London, using the BioCAT 18-IDD beamline at the APS have discovered the molecular basis of this fundamental property of muscle function. Their work was the cover article for the issue of Cell magazine in which it was published. Understanding exactly how muscle fibers work helps lay the groundwork for the development of new treatments for muscle disorders as well as enhancing the understanding of athletic performance.
The researchers gleaned these insights into muscle function by using the BioCAT beamline to study a key muscle protein called myosin. The group characterized the interaction of myosin with actin, another protein found in muscle. Actin and myosin are arranged within muscle fibers in alternating layers of filaments. The actin and myosin layers are connected by “myosin motors,” which, during a muscle contraction, cause the layers to slide over each other in opposing directions, similar to the way oars dipped into water propel a row boat forward. The shortening of a muscle fiber is thought to correlate with the myosin and actin sliding over each other in this manner. When several fibers contract together, a muscle contraction takes place.
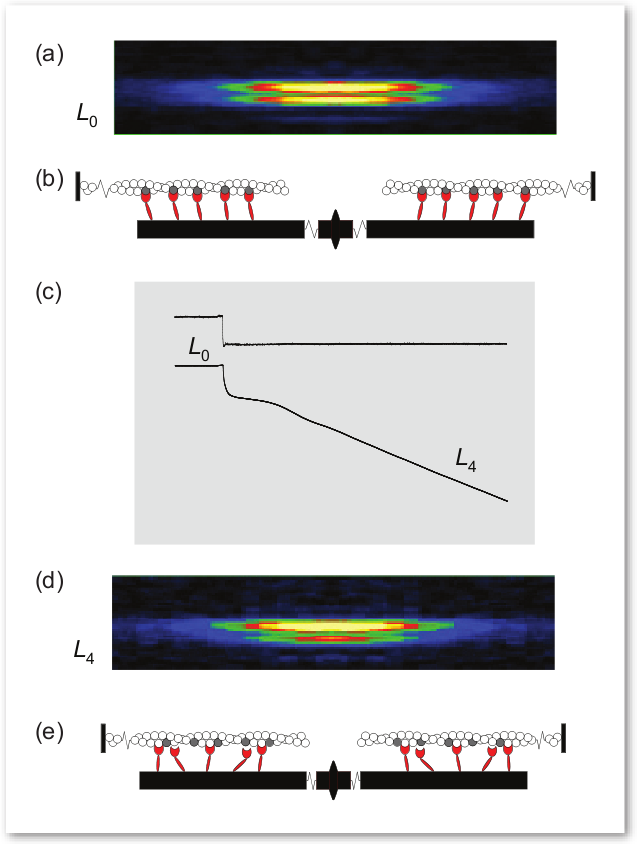
These researchers showed, for the first time, that muscle fibers not only shorten more slowly with a heavier load, but that the number of myosin motors attached to actin increases directly with increased load, while the size of their movement remains almost unchanged. This explanation more accurately depicts muscle performance than the previously accepted explanation.
The researchers conducted their experiments on single intact fibers, about 5 mm in length, dissected from frog muscle. Electrodes were used to deliver pulses of electric current to the muscle fibers that simulated what would take place in a real-life setting. In this way, the researchers were able to record the fiber contractions under various conditions. X-ray diffraction data were then collected from the fibers, which enabled the researchers to visualize and quantify the movement of attached myosin motors as load increased (Fig. 1).
Simultaneous mechanical measurements enabled the researchers to determine that the number of the attached myosin motors increased in proportion to the increase in load, while the force exerted by each of the myosin motors remained constant regardless of the load. This was in contrast to what had been previously thought—that the force of each myosin motor was higher when the load was higher. These researchers found that it was the increased number of connecting myosin motors that allows the muscle fiber to bear greater weight, not an increase in force within each myosin motor.
The researchers also found that detachment of a myosin motor from actin depended heavily on the motor’s shape or conformation as a muscle progressed through a contraction. The change in shape allowed myosin motors to detach more quickly from actin while bearing lighter loads and remain attached while bearing heavier loads, a finding that gives a molecular explanation for the high efficiency of this motor.
See: Gabriella Piazzesi, Massimo Reconditi, Marco Linari, Leonardo Lucii, Pasquale Bianco, Elisabetta Brunello, Valérie Decostre, Alex Stewart, David B. Gore, Thomas C. Irving, Malcolm Irving, and Vincenzo Lombardi, “Skeletal Muscle Performance Determined by Modulation,” Cell 131, 784 (November 16, 2007). DOI 10.1016/j.cell.2007.09.045
Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Based on an APS press release by Emma Hitt.