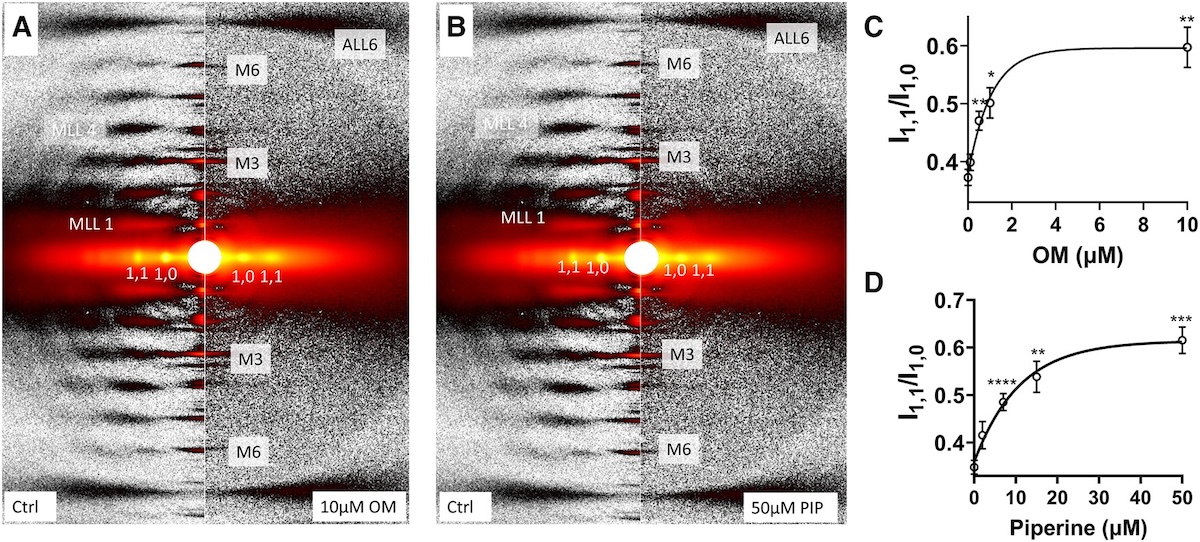
Myosin-based thick-filament regulation is now known to be critical for muscle contraction with myosin dysregulation found in hypertrophic and dilated cardiomyopathies but many details of thick filament regulation remain to be discovered. Myosin ATPase assays have demonstrated that under relaxed conditions, myosin may reside either in a high-energy-consuming disordered-relaxed (DRX) state available for binding actin to generate force or in an energy-sparing super-relaxed (SRX) state unavailable for actin binding. X-ray diffraction studies have shown that the majority of myosin heads are in a quasi-helically ordered OFF state in a resting muscle and that this helical ordering is lost when myosin heads are turned ON for contraction. It has been assumed that myosin heads in SRX and DRX states are equivalent to the OFF and ON states, respectively, and the terms have often been used interchangeably. In this study, the authors used X-ray diffraction and ATP turnover assays to track the structural and biochemical transitions of myosin heads, respectively, induced with either omecamtiv mecarbil (OM) or piperine in relaxed porcine myocardium. We find that while OM and piperine induce dramatic shifts of myosin heads from the OFF to the ON state, there are no appreciable changes in the population of myosin heads in the SRX and DRX states in both unloaded and loaded preparations. Our results show that biochemically defined SRX and DRX can be decoupled from structurally defined OFF and ON states. While previously thought to be synonymous, this study finds that biochemical and structural thick-filament disengagements have distinct properties and should be investigated as independent phenomena. Understanding the details of thick-filament regulation will be of great relevance to defining sarcomere-level dysfunction in myopathies and understanding and better designing sarcomere therapies aimed at reversing them for treatment of cardiomyopathy.
See: Vivek P Jani, Taejeong Song, Chengqian Gao, Henry Gong, Sakthivel Sadayappan, David A Kass, Thomas C Irving, Weikang Ma, “The structural OFF and ON states of myosin can be decoupled from the biochemical super- and disordered-relaxed states,” PNAS Nexus 3 (2), pgae039 (2024). DOI: 10.1093/pnasnexus/pgae039. PMCID: PMC10849796