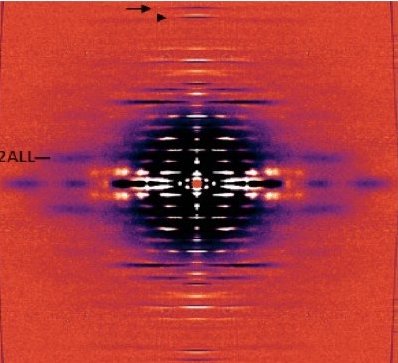
Force in muscle is generated by interactions between myosin motor proteins arranged in thick filaments and their binding sites on thin filaments made mostly of the protein actin. Nebulin is a giant actin-binding protein in skeletal muscle that is found along the length of the thin filaments but up to now, not much was known about what it does to affect muscle function. Mutations in the nebulin protein cause the muscles in patients with nemaline myopathy disease to be weak in patients, suggesting that properly functioning nebulin is important to generate force. This condition can be recapitulated in genetically engineered mice who have mutated nebulin or entirely lack nebulin so that the muscles in these animals also show weakness. Investigators from the University of Arizona and the Illinois Institute of Technology used the BioCAT beamline 18ID to study mice entirely lacking nebulin to discover the effects of nebulin on the nanoscale structure of muscle.
Using X-ray diffraction, they found that the thin filaments were found to be 3-fold less stiff in nebulin-deficient muscles and that the action of other proteins that normally function to turn on and off the thick filament were impaired. As a consequence, fewer myosin motors are recruited to their actin binding sites and the muscle generates less force. So it appears that nebulin stiffens the thin filaments and is necessary for generating physiological levels of force.
See: Balazs Kiss, Eun-Jeong Lee, Weikang Ma, Frank Li, Paola Tonino, Srboljub M. Mijailovich, Thomas Irving, and Henk Granzier. Nebulin stiffens the thin filament and augments cross-bridge interaction in skeletal muscle. Proc Natl Acad Sci U S A. 2018