Netrins are a conserved class of proteins involved in synaptic connectivity of the nervous system in bilaterian animals. They act as secreted guidance cues, with the unique ability to exert repulsive and attractive responses on growing axons. They are also known to be involved in cell proliferation, migration and differentiation, and are therefore targets for treating cancer and insulin resistance. During axon growth and cell migration, the presence of the receptor Uncoordinated-5 (UNC-5) on target cells results in repulsion. However, the exact mechanism involved in the induction of repulsive forces has been relatively unknown due to the lack of biochemical and structural information about these systems. Researchers at the University of Chicago and Stanford University, in collaboration with BioCAT staff, showed that UNC-5 is a heparin-binding protein, determined its structure bound to a heparin fragment, and could modulate UNC-5–heparin affinity using a directed evolution platform or structure-based rational design.
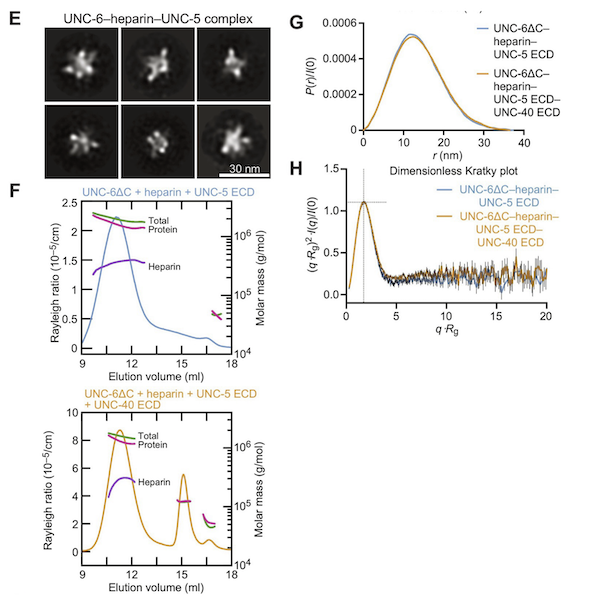
A question they needed to answer was whether the heparin + UNC-6–mediated UNC-5 oligomers are an ordered and well-defined molecular species or (ii) whether these oligomers are simply a result of UNC-6 and UNC-5 decorating the long, linear heparin chains. They used negative-staining electron microscopy, multi-angle light scattering (MALS) and small-angle x-ray scattering (SAXS) to visualize the UNC-5–heparin–UNC-6 complex. The EM images and class averages consistently show a large, globular complex as opposed to long or thin chains, a description consistent with models from the SAXS data where the pair distribution [P(r)] plot and molecular shape analysis both indicate a strongly globular complex. Given the multi-domain nature of the system and the flexibility of the UNC-5 ectodomain, this was not expected and indicates that the UNC-6–heparin–UNC-5 complex has a relatively defined structure with ordered geometry, which may represent a signaling-competent conformation on the cell surface.
The work presented here establishes a structural and molecular model that provides context for much of the existing netrin literature, and may serve as a stepping-stone toward an atomic-resolution structure of the complete complex.
See: Jessica M. Priest, Ev L. Nichols, Robert G. Smock, Jesse B. Hopkins, Juan L. Mendoza, Rob Meijers, Kang Shen, and Engin Özkan. Structural insights into the formation of repulsive netrin guidance complexes. Science Advances 2024 Vol 10, Issue 7, DOI: 10.1126/sciadv.adj8083. PMCID: PMC10871540