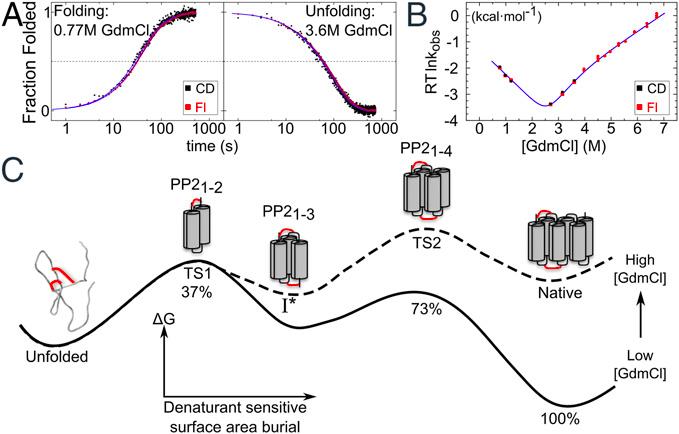
The physical basis of protein folding stability and cooperativity remains a topic of great interest. Folding of globular proteins is generally assumed to be driven by energetically favorable burial of hydrophobic groups and that early development of secondary structure increases the cooperativity of folding. The Sosnick group at the University of Chicago examines these assumptions in a protein (snow flea antifreeze protein (sfAFP) that is striking in its dearth of hydrophobic burial and its lack of α and β structures, while having a low sequence complexity with 46% glycine. The interior of the protein is filled only with backbone H-bonds between six polyproline 2 (PP2) helices. Unexpectedly, the protein folds in a kinetically two-state manner and is moderately stable at room temperature, similar behavior to that observed for typical globular proteins having α and β structures and a hydrophobic core. Hence, these features are not necessary for folding cooperativity and stability. This enigma forces a reexamination of the possible combination of factors that can stabilize a protein. The authors propose that a major part of the stability arises from the unusual match between residue-level PP2 dihedral angle bias in the unfolded state and PP2 helical structure in the native state. Additional stabilizing factors that compensate for the dearth of hydrophobic burial include shorter and stronger H-bonds, and increased entropy in the folded state. These results extend our understanding of the origins of cooperativity and stability in protein folding, including the balance between solvent and polypeptide chain entropies.
See: Zachary P. Gates, Michael C. Bax, Wookyung Yu, Joshua A. Riback, Hui Li, Benoît Roux, Stephen B. H. Kent, and Tobin R. Sosnick. PNAS 2017 114: 2241–2246.