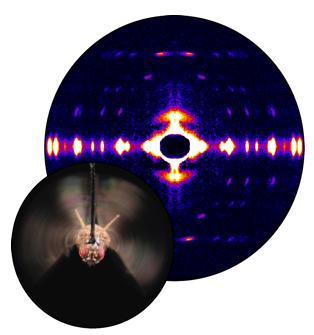
Fruit flies beat their wings faster than their cellular powerplants can generate the energy needed for flapping. To resolve this energetic discrepancy, researchers from the California Institute of Technology, the Illinois Institute of Technology, and the University of Vermont used the BioCAT beamline 18-ID at the APS to obtain a series of x-ray photographs that revealed the flies’ secret: A muscle protein used to power wings acts like a spring, storing energy while stretched before snapping back. Not only did this finding surprise researchers who study muscle, but the results might also help scientists better understand the human heart.
Drosophila, the fruit fly, beats its wings up and down once every 5 milliseconds. Two layers of muscle control this action: when one layer contracts, it stretches the other. The stretched muscle is then primed to contract; when it does, it stretches the first layer and completes the cycle. These cycles occur faster than nerve impulses can stimulate them; hence, the muscles themselves must keep the beat going.
To find out how muscles do this, the research team needed to visualize the proteins that make muscle contract. Within the flight muscle cells, two proteins cooperate to do this. Myosin proteins have flexible heads and long tails that bundle together. The heads grab filaments made of the protein actin that ultimately connect at either end to opposite cell walls. Like a bunch of hands pulling on a rope, the myosin heads drag actin inward, making the muscle cell shorter. Methods to visualize what proteins look like often require purifying the protein or killing the organism in which it exists. Not only did the team want to keep the flies alive, but they wanted to watch myosin and actin in action. Short bursts of APS’s high-intensity x-rays allowed them to achieve both goals.
The researchers tethered flies inside a box (a “flight simulator”) and used air and moving lights to convince the bugs that they were buzzing around. Then the team took x-ray snapshots of the proteins responsible for beating the wings. They synchronized the beam’s shutter speed with the wing beat’s frequency. Much like a strobe light reveals the status of a dancer instantaneously, synching the wing beat to the synchrotron x-rays allowed the scientists to view the positions of the muscle proteins at one point in time. The team put together a series of these images taken at different points of the beat to create a stop-action film of the protein movements within muscle cells.
After exposure in the beam, myosin and the other muscle components diffract x-rays onto a detector in specific patterns. From this pattern, researchers can tell if myosin is grabbing onto the actin filament, pulling, or releasing its grasp. In contracted muscle, the researchers saw myosin dragging the actin filament as expected.
But when the muscles relaxed, the team saw an unanticipated movement. Instead of completely releasing its grasp as myosin does in other relaxed muscles, the myosin briefly let go, but then snagged the filament again. And the bundle of myosin tails—long believed to be stiff for pulling during contractions—stretched a bit, due to the second layer of contracting flight muscle. This finding suggested to the researchers that myosin stores elastic energy in its tail that can be used in the next contraction, much like a stretched spring holds onto energy and releases it when the spring recoils. The stretching also appears to stimulate the myosin and actin filaments to again contract the muscle.
In addition to this novel finding, the technological advancement that allows scientists to view the composition of a living muscle will now let them study muscle contraction in greater detail. Fruit flies are well suited for genetic studies that might eventually illuminate how human heart muscle, which is similar to a fly’s muscle, can fail when diseased.
See: M. Dickinson, G. Farman, M. Frye, T. Bekyarova, D. Gore, D. Maughan, and T. Irving, “Molecular Dynamics of Cyclically Contracting Insect Flight Muscle In Vivo,” Nature 433, 330 (20 January 2005)
This work was supported by the National Institutes of Health. The APS is supported by the U.S. Department of Energy. Bio-CAT is an NIH-supported Research Center. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31-109-ENG-38.
Based on an APS press release by Mary Beckman.