Many of us are familiar with mad cow disease–the neurodegenerative disease caused by prions. Although they have a similar name, the less familiar prion- like domains (PLDs) refer to something different–unique, low-complexity regions of proteins that are capable of regulating gene expression and affecting important cellular processes. Prion-like domains have become a topic of interest because of their connection with a variety of debilitating brain diseases, such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia. In fact, mutations in PLDs of some genes have been shown to cause neurodegenerative disease. For example, mutations in PLDs of the genes hnRNPA2B1 and hnRNPA1 can cause the neurodegenerative disorders ALS and multisystem proteinopathy. A recent study using data obtained at BioCAT completed a comprehensive biophysical investigation of PLDs in the protein hnRNPA1 to uncover the major behavioral and structural features of these domains. This meaningful work may lead to discoveries that can help individuals living with such neurodegenerative diseases.
Amyotrophic lateral sclerosis is a devastating disease of the nervous system that affects the brain and spinal cord. Patients often present with symptoms such as slurred speech, muscle twitching, and muscle weakness. Patients lose total control of muscle function as the disease progresses, resulting in a fatal inability to perform everyday tasks such as eating, breathing, moving, and speaking. Age of onset tends to occur later in adult life; at present, there is no cure.
Although the cause of ALS is largely unknown, genetic mutations in PLDs is known to be involved. PLDs have been shown to impact a variety of important cellular processes, such as cell division and understanding how cells respond to stress. The proteins hnRNPA2B1 and hnRNPA1 serve important functions in the processing and stabilization of mRNA. More broadly, PLDs can drive the aggregation of proteins within cells.
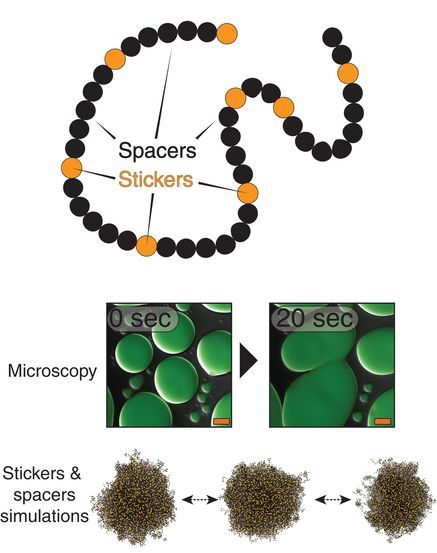
Since PLDs have such a palpable relevance to medicine and brain health, investigations into their behavior and structure are highly valuable. Seeking to better understand how PLDs behave and affect molecular aggregation, the authors in this study, from St. Jude Children’s Research Hospital, Washington University in St. Louis, and Washington University School of Medicine, found that the temperature-dependent compaction of molecules within a liquid solution is determined by the number of aromatic residues in PLDs. The uniform patterning of these aromatic residues promotes a phenomenon known as liquid-liquid phase separation while preventing aggregation. Liquid-liquid phase separation is a physical process that leads to the formation of two co-existing liquid phases (a dilute and a dense phase), which is the underlying process for the formation of many non-membrane bound cellular compartments. The authors additionally put forth an impressive stickers-and-spacers model (adapted from the associative polymer field) that can be used to make predictions regarding the behavior of PLDs. This study utilized multiscale simulations, nuclear magnetic resonance spectroscopy, and small-angle x-ray scattering (SAXS) to uncover behavioral and structural features of PLDs (Fig. 1). The SAXS research was carried out using high-brightness x-rays at the BioCAT beamline at the APS. The high brightness x-rays were essential to characterize small differences between structural features of different mutants of the PLD.
The data generated from this work will enable more precise characterization of PLDs within cells, which will in turn provide the ability to predict how PLDs form and dissolve condensates in response to different circumstances (e.g., protein concentration, conditions in cells). Given that PLDs appear to have an important role in the maintenance of brain health, the significant insights into how phase behavior and structure of PLDs are coupled may allow for the development of PLD-targeted therapeutics that can help patients with ALS and other debilitating neurodegenerative disorders.
See: Erik W. Martin, Alex S. Holehouse, Ivan Peran, Mina Farag, J. Jeremias Incicco, Anne Bremer, Christy R. Grace, Andrea Soranno, Rohit V. Pappu, Tanja Mittag. “Valence and patterning of aromatic residues determine the phase behavior of prion-like domains,” Science 367, 694–699 (2020), DOI: 10.1126/science.aaw8653