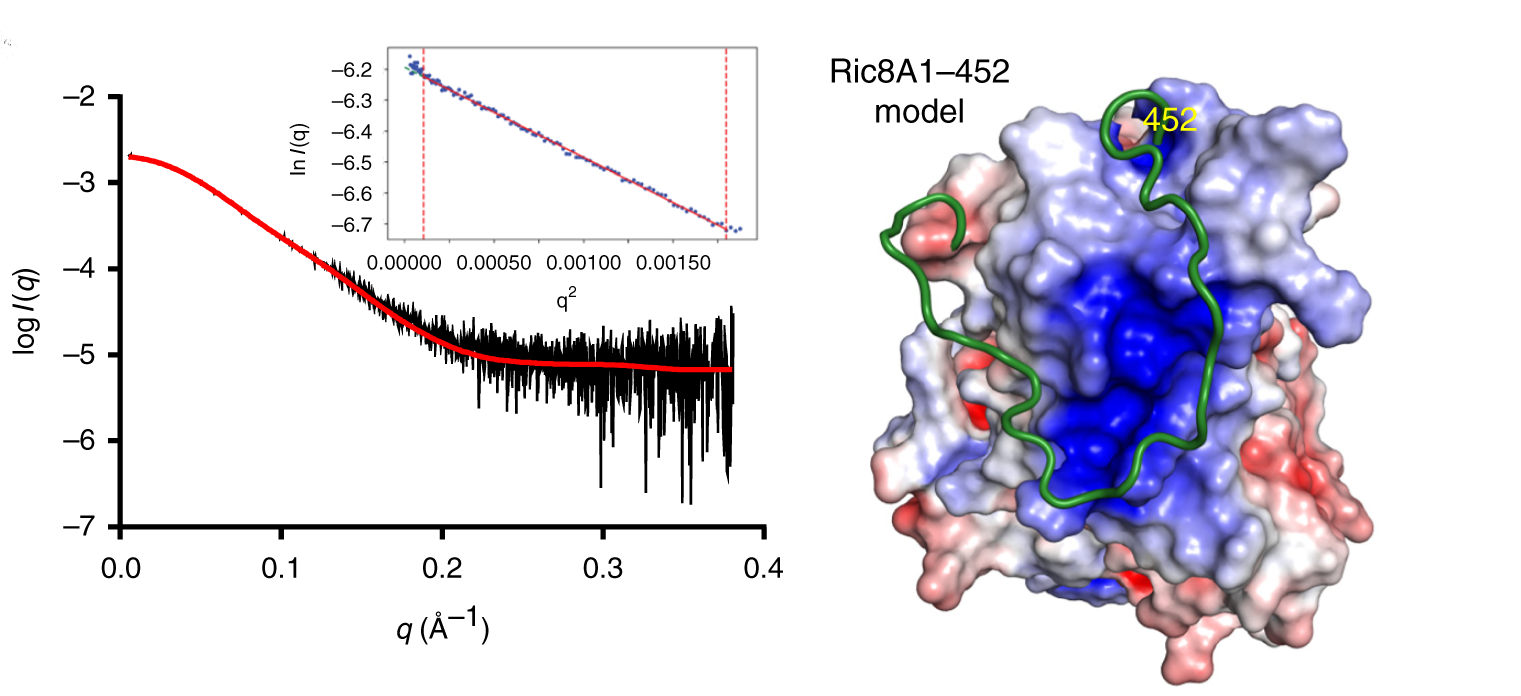
G-protein signaling has been the dominant theme in the Artemyev lab and their recent work specifically addresses Ric8A (Resistance to inhibitors of cholinesterase 8A) structure and function. Ric8A is a well-known regulator of G-protein biology and belongs to a class of proteins different from the G protein-coupled receptors (GPCRs), which act via interactions with monomeric Gα subunits as opposed to heterotrimeric Gαβγ proteins. SAXS was used in combination with crystallography and biochemical studies to show that the flexible C-terminal tail is important for the overall stability of Ric8A and the function as a guanine nucleotide exchange factor (GEF). The crystal structure revealed that Ric8A belongs to a functionally diverse class of proteins with what is known as an armadillo-fold (ARM) characterized by two layers of alpha helices arranged in a right handed superhelix. Ric8A diverged from the class in terms of the number of ARM repeats (8 as opposed to 10) and is further followed by a flexible region spanning ~ 70 residues.
Differential scanning fluorimetry (DSF) was used to determine that a construct without the C-terminal 70 amino acids was significantly less stable which suggests that this flexible tail region is involved in intramolecular interactions and it may allosterically promote its binding to Gα. Molecular dynamics simulations were used to model experimental SAXS data and it was clear that an acidic stretch of amino acids from the proximal region of the C-terminal tail was involved in an extensive network of stabilizing interactions with the core of Ric8A. Using SAXS data obtained from a construct containing both the proximal and distal tail regions the distal part of the C-terminal tail was shown to have extended conformations and to have no interactions with the Ric8A core, while the biochemical studies showed that this part of the tail is essential for the complex formation with Gα. Information from a variety of complementary biochemical and biophysical techniques was instrumental in clarifying the true mechanism of Ric8A and in delineating the similarities and differences between Ric8A and canonical GPCRs.
See: Srivastava, D., L. Gakhar, and N.O. Artemyev, “Structural underpinnings of Ric8A function as a G-protein alpha-subunit chaperone and guanine-nucleotide exchange factor”. Nat Commun, 2019. 10(1): p. 3084. PMCID: PMC6625990, DOI: 10.1038/s41467-019-11088-x