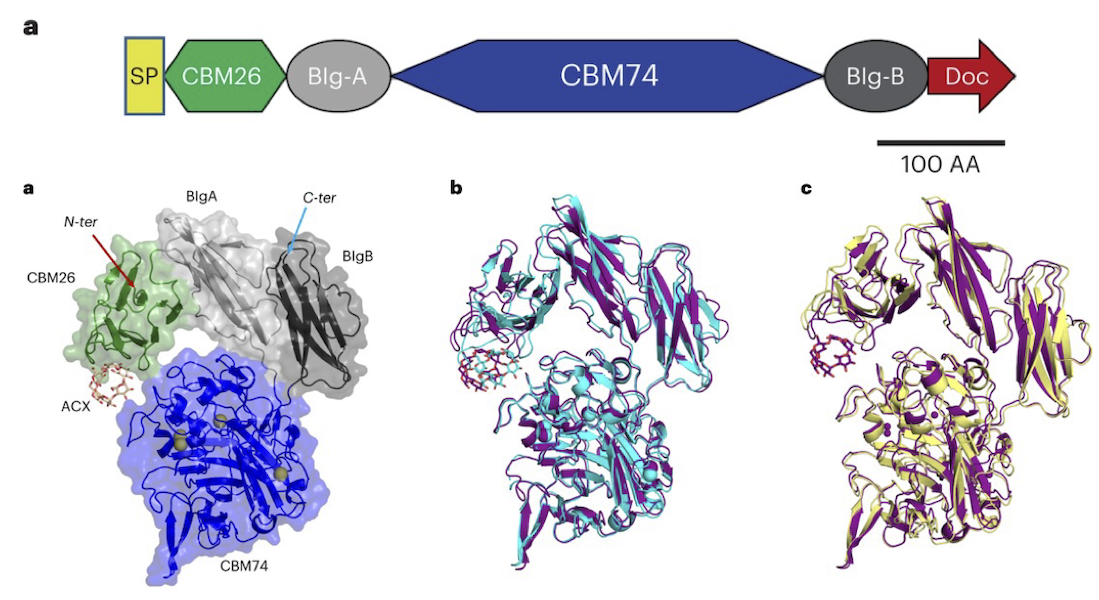
The ways in which starches, in particular digestion-resistant starches, are accommodated by gut bacteria remains relatively poorly understood at the molecular level. Digestion-resistant starches are accessed by specialized gut bacteria with specific carbohydrate-binding systems. One such system is the Ruminococcus bromii Starch Adherence System member 6 (Sas6), which consists of two starch-specific carbohydrate-binding modules from family 26 (RbCBM26) and family 74 (RbCBM74). The authors present a structural and functional characterization by crystallography, SAXS, native mass spec and other methods of one such system from Ruminococcus bromii (Sas6). The crystal structure of Sas6 showed that the RbCBM74 starch-binding groove complements the double helical α-glucan geometry of amylopectin, suggesting that this module selects this feature in starch granules. The overall structure was compact – both in crystals and in solution, as measured by size-exclusion chromatography-coupled SAXS (SEC-SAXS) – stabilized by significant hydrogen bonding networks between the domains. SEC-SAXS experiments were performed at BioCAT. Isothermal titration calorimetry and native mass spectrometry demonstrate that RbCBM74 recognizes longer single and double helical α-glucans, while RbCBM26 binds short malto-oligosaccharides. Bioinformatic analysis supports the conservation of the amylopectin-targeting platform in CBM74s from resistant-starch degrading bacteria. Together, these data allowed the authors to elucidate the starch granule recognition mechanism, providing foundational work for both engineering of starch-degrading systems as well as gut-microbiome focused health applications.
See: Amanda L Photenhauer, Rosendo C Villafuerte-Vega, Filipe M Cerqueira, Krista M Armbruster, Filip Mareček, Tiantian Chen, Zdzislaw Wawrzak, Jesse B Hopkins, Craig W Vander Kooi, Štefan Janeček, Brandon T Ruotolo, Nicole M Koropatkin. The Ruminococcus bromii amylosome protein Sas6 binds single and double helical α-glucan structures in starch. Nat. Struct. Mol. Biol. 2024 Feb;31(2):255-265. DOI: 10.1038/s41594-023-01166-6. PMCID: PMC11081458