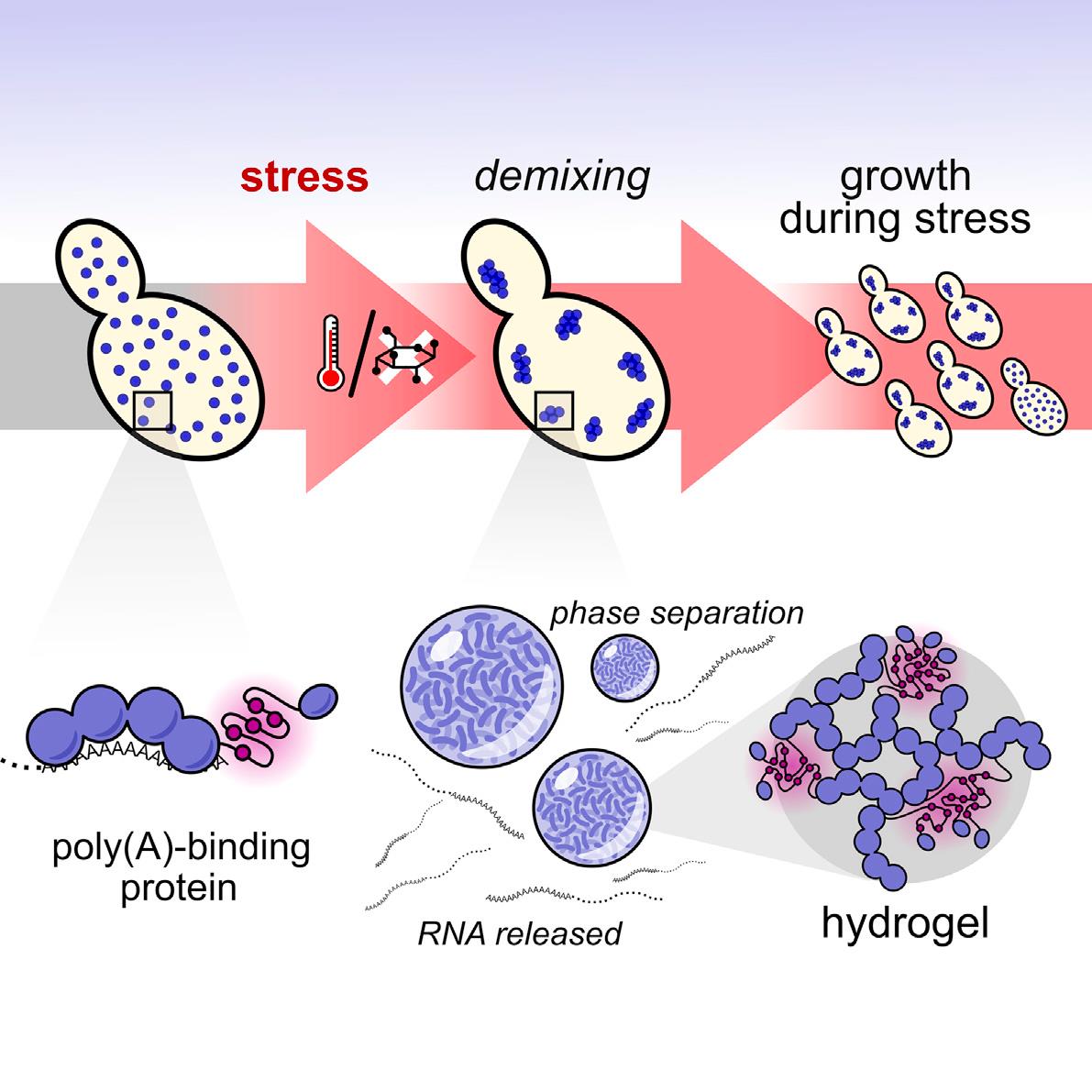
In eukaryotic cells, diverse stresses trigger coalescence of various RNA binding proteins into so-called stress granules. In vitro, stress-granule associated proteins have been observed to demix to form liquids, hydrogels, and other assemblies. Demixing of an abundant RNA-binding protein into hydrogel droplets, triggered by stress-associated physiological conditions, appears to promote cell fitness during stress.
Here, a U Chicago based team lead by D. Allan Drummond and Tobin Sosnick showed that poly(A)-binding protein (Pab1 in yeast), a defining marker of stress granules, phase separates and forms hydrogels in vitro upon exposure to physiological stress conditions. Other RNA-binding proteins depend upon low-complexity regions (LCRs) or RNA for phase separation. Based on unique evolutionary patterns, the authors created LCR mutations, which systematically tune its biophysical properties and Pab1 phase separation in vitro and in vivo. Mutations that impede phase separation reduced organism fitness during prolonged stress. Such mutations reduce the thermal and pH sensitivity of Pab1’s demixing and, hence, reduce fitness during growth at high temperature and during energy depletion, indicating that demixing is adaptive. Together, the results illuminate a uniquely complete path from evolved sequence features, to phase separation, to stress-triggered demixing, and finally to organism fitness during stress. SEC_SAXS using the BioCAT beamline 18ID at the Advanced Photon Source of the unstructured P domain of the poly A binding protein allowed examination of how its compaction related to its hydrophobicity, as tuned by various mutations, an important component of this model.
See: Joshua A. Riback, Christopher D. Katanski, Jamie L. Kear-Scott, Evgeny V. Pilipenko, Alexandra E. Rojek, Tobin R. Sosnick, and D. Allan Drummond. 2017, Cell 168, 1028–1040.