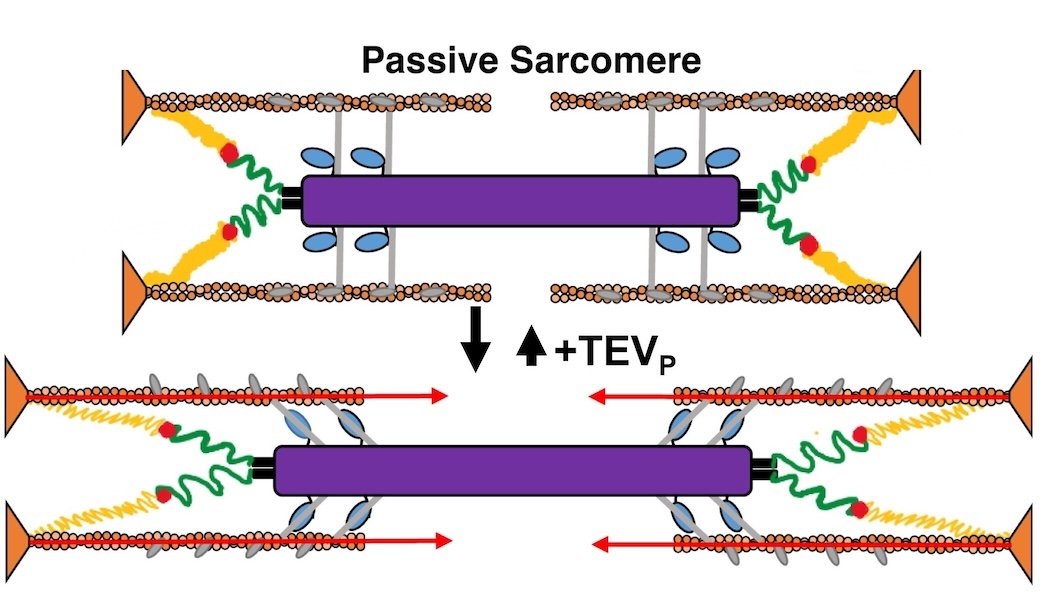
Muscles can produce more force when stretched to a longer length at the same level of activating calcium, a poorly understood phenomenon known as myofilament length dependent activation (LDA). It was suggested several years ago that passive force generated by the giant elastic protein titin could be the length sensor behind this phenomenon, but direct evidence has been lacking. Investigators from the University of Muenster in Germany, Northern Arizona University, University of British Columbia, and the Illinois Institute of Technology used a mouse model with a cleavage site inserted into the titin protein allowing titin to be enzymatically cut in the mature tissue (with TEVp protease) to make it ineffective. Small-angle X-ray diffraction of muscle from these mice with and without cleavage showed that titin cleavage diminished the changes in length-dependent structural signatures (“priming”) associated with LDA. Strikingly, a titin-sensitive, length-dependent structural changes were also seen in thin filaments, which seems only possible if there are bridging structures between the thick and thin filaments in resting muscle, potentially comprised of myosin-binding protein C. These experiments firmly established titin as the length sensor in LDA and showed that LDA involves structural changes in both thick and thin filaments.
See: Anthony L Hessel, Weikang Ma, Nicole Mazara, Paige E Rice, Devin Nissen, Henry Gong, Michel Kuehn, Thomas Irving, Wolfgang A Linke. Titin force in muscle cells alters lattice order, thick and thin filament p rotein formation. Proc Natl Acad Sci U S A. Nov 29;119(48):e2209441119 (2022). DOI: 10.1073/pnas.2209441119. PMCID: PMC9860331.