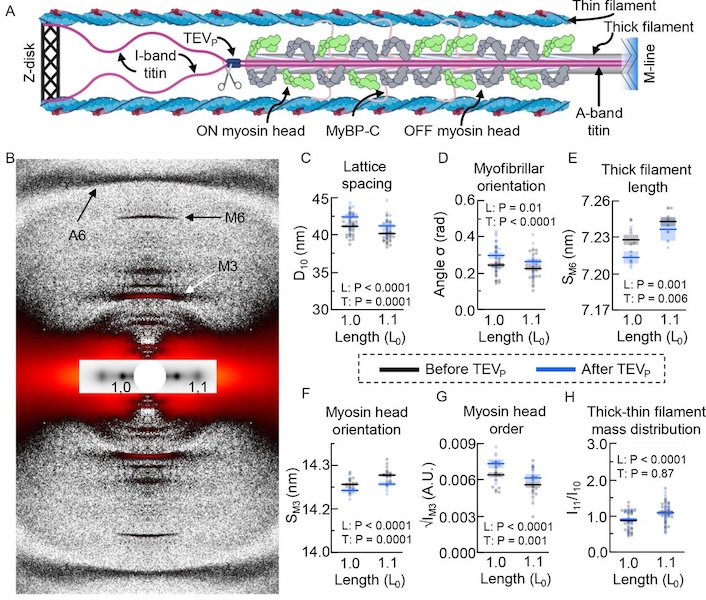
The Frank-Starling Law of the Heart states that the heart’s stroke volume increases with greater preload due to increased venous return, allowing the heart to adapt to varying circulatory demands. At the molecular level, increasing preload increases sarcomere length (SL), which alters structures within the sarcomere that are correlated to increased calcium sensitivity upon activation. The titin protein, spanning the half-sarcomere acts as a spring in the I-band, applies a SL-dependent passive force on the myosin containing thick filaments changing its structure and functional properties. Altered titin-based forces play a crucial role in the etiology of many cardiomyopathies; however, the disease state obscures titin’s role, impeding therapeutic solutions.
The authors studied titin’s specific role using the titin cleavage (TC) mouse model, where a tobacco-etch virus protease (TEVP) recognition site is inserted into distal I-band titin and allows for rapid, specific cleavage of titin in an otherwise-healthy sarcomere. Titin cleavage decreases myocardial passive force and stiffness while altering cardiomyocyte tensegrity. Structural changes in the sarcomere as a function of stretch following 50% titin cleavage were monitored using small-angle X-ray diffraction. Increasing titin-based forces at longer SLs could stretch the cardiac thick filament, accompanied by a transition of some myosin heads from an inactive OFF to an active ON confirmation. This mechanism may increase the myosin head’s ability to form crossbridges, contributing to the Frank-Starling effect. The length of the thick filament, monitored by the spacing of the M6 meridional reflection (SM6) increases at longer SL and decreases with 50% titin cleavage. Other indicators of myosin head OFF-to-ON transitions with increasing SL before cleavage, including increasing M3 spacing and decreasing myosin layer line intensity, were blunted with 50% titin cleavage while there was no change in equatorial intensity ratio, I11/I10 indicating that there were no changes in the radial position of myosin heads, while myosin heads were still able to reorient. Interesting, these changes in thick filament length were accompanied by changes in thin filament length as measured by the A6 actin reflection spacing, that were also blunted by titin cleavage. These results support earlier suggestions that there must be a communication pathway, perhaps small numbers of strongly bound crossbridges, or myosin binding protein C. that transmits strain in the thick filaments to the thin filaments. The authors concluded that reducing titin-based forces blunts structural changes in both thick and thin filaments while leaving the length-dependent OFF-to-ON transition mechanism intact, indicating a clear role for titin in the Frank-Starling mechanism.
See: Anthony L. Hessel, Michel N. Kuehn, Nichlas M. Engels, Devin L. Nissen, Johanna K. Freundt, Weikang Ma, Thomas C. Irving, and Wolfgang A Linke. Titin-based force regulates cardiac myofilament structures mediating length-dependent activation Circ Res. 2024 Apr 12;134(8):1026-1028. Accepted ahead of publication.