Diaphragm unloading during mechanical ventilation is an important clinical problem. The diaphragm is the main muscle of respiration and contracts during each breath, thereby changing the anatomic configurations of the chest wall so that air flows into the lungs. Increased diaphragm loading is associated with diaphragm fiber contractile dysfunction, atrophy and injury. Whereas these effects of increased loading on the diaphragm take months or years to develop, the effects of decreased loading, as occurs when ICU patients are mechanically ventilated, occur extremely rapidly, within hours. For ICU patients with respiratory failure this mechanical ventilation is considered a life-saving supportive therapy. However, mechanical ventilation, with its attendant diaphragm muscle inactivity, can cause rapid diaphragmatic weakness, leading to difficulties in discontinuing this ventilatory support (i.e. weaning failure). Weaning failure is a major clinical problem; it is encountered in >30% of mechanically ventilated ICU patients, it consumes ~40% of all ICU resources, and, importantly, patients experiencing weaning failure are at much higher risk for death due to pneumonia and airway trauma. These issues came to the fore with the large numbers of mechanically ventilated ICU patients during the COVID 19 pandemic.
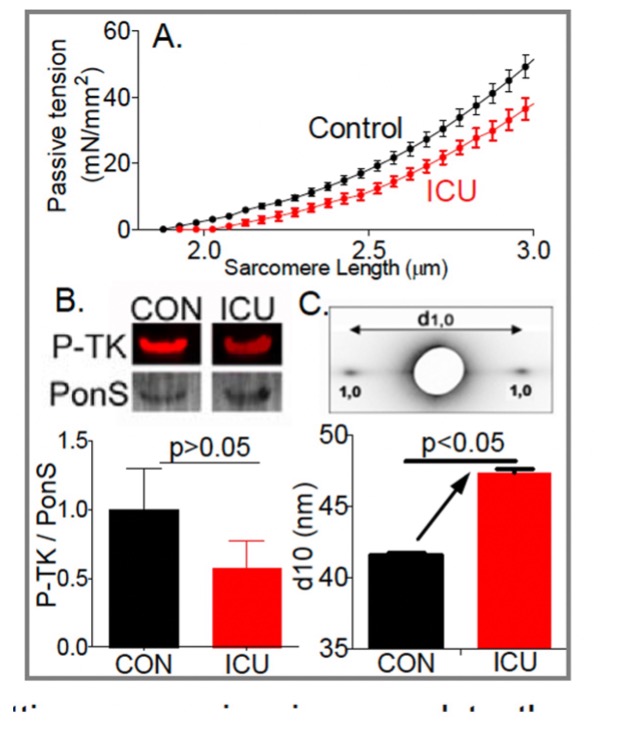
Not known is which structures sense the mechanical unloading of the diaphragm and set in motion the molecular cascades leading to atrophy. Here we test the hypothesis that the mechano-sensor protein is titin, a giant elastic protein connecting Z-disks and the thick filaments in the A-band. Titin acts as a myofilament lattice stabilizer via its passive force running obliquely to the thin and thick filaments. This results in the production of radial as well as longitudinal forces in the lattice when muscle is stretched, with the radial forces tending to pull the thin filament closer to the thick filament. This increases the likelihood of myosin attaching to the thin filament, thereby elevating the Ca2+ sensitivity of force generation at long sarcomere length. Our preliminary X-ray diffraction data show that the reduced titin stiffness in diaphragm fibers from ICU patients expands the myofilament lattice and that this can explain the observed reduction in calcium sensitivity. In the proposed work we determine whether myofilament lattice spacing is affected in diaphragm fibers from mechanically ventilated rats and study whether this effect is dependent on titin-based passive tension. To critically test that myofilament lattice expansion is causal to the reduced calcium sensitivity we will study whether compression of the lattice back to control values (by using Dextran T-500) restores the calcium sensitivity of force in ICU diaphragm fibers. Together with transcriptome and protein work, we anticipate establishing that titin-based mechano-sensing modulates the development of diaphragm atrophy and weakness during mechanical ventilation. We will also study the mechanistic basis for diaphragm weakness in mechanically ventilated ICU patients using, for the first time, diaphragm fibers isolated from biopsies of these patients. The goal is to investigate whether the findings of animal studies extrapolate to patients. The obtained results will be compared to those from rectus abdominus muscle to determine to what extent the observed changes are specific to the diaphragm or part of generalized muscle weakness.
Interactions between BioCAT staff and this group were very important in developing the techniques for studying human biopsy samples from diaphragm muscle. The very small samples would be difficult to study on any beamline other than 18ID. The proposed experiments will also benefit from the muscle biophysics support laboratory at the beamline where parallel biochemical assays can be performed in the same experimental sessions as the X-ray experiments.
See: M. van den Berg , Z. Shi, W. Claassen, P. Hooijman P, C. Lewis, J. Andersen, R. van der Pijl RJ, S. Bogaards, S. Conijn, E. PetersL, L. Begthel LPL, B. Uijterwijk, J. Lindqvist, P. Langlais, A. Girbes, S. Stapel S, H. Granzier, K. Campbell, W. Ma, T. Irving, D. Hwee, J. Hartman, F. Malik, M. Paul, A. Beishuizen, J. Ochala, L. Heunks L, C. Ottenheijm Super-relaxed myosins contribute to respiratory muscle hibernation in mechanically ventilated patients. Sci Transl Med. 2024 Jul 31;16(758):eadg3894. doi: 10.1126/scitranslmed.adg3894. PMCID: PMC11586073