Targeting Cancer at the Level of DNA Expression
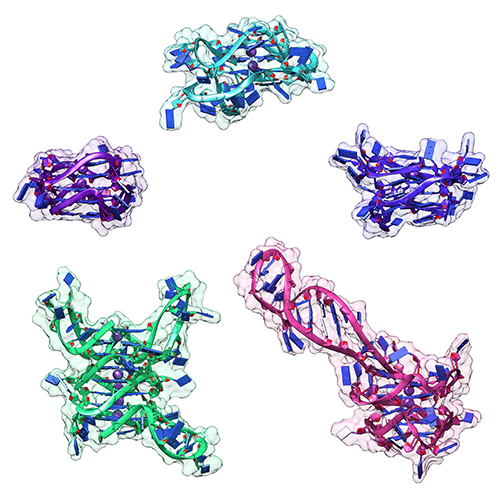
The last 20 years have brought a revolution in targeted therapies for cancer. Small-molecule inhibitors and monoclonal antibodies that target a specific aberrant protein in tumors have provided cancer patients with treatments that are associated with fewer side effects and longer survival than conventional chemotherapy. This has been, in large part, the result of intensive research into the role of oncogenes in cancer development. Oncogenes are normal cellular genes that have become mutated in such a way that they aberrantly promote the uncontrolled cell growth seen in cancer. They are often proteins involved in growth control or activation of cellular signaling; inhibiting these mutated proteins has proven to be effective in stopping the growth of many cancers. Research by a team from the Brown Cancer Center at the University of Louisville in Kentucky using the U.S. Department of Energy’s Advanced Photon Source (APS) and published in the journal Nucleic Acids Research promises to extend these treatment possibilities to control these oncogenes at the gene …