NUCLEOTIDE-INDUCED ASYMMETRY WITHIN ATPASE ACTIVATOR RING DRIVES σ54-RNAP INTERACTION AND ATP HYDROLYSIS
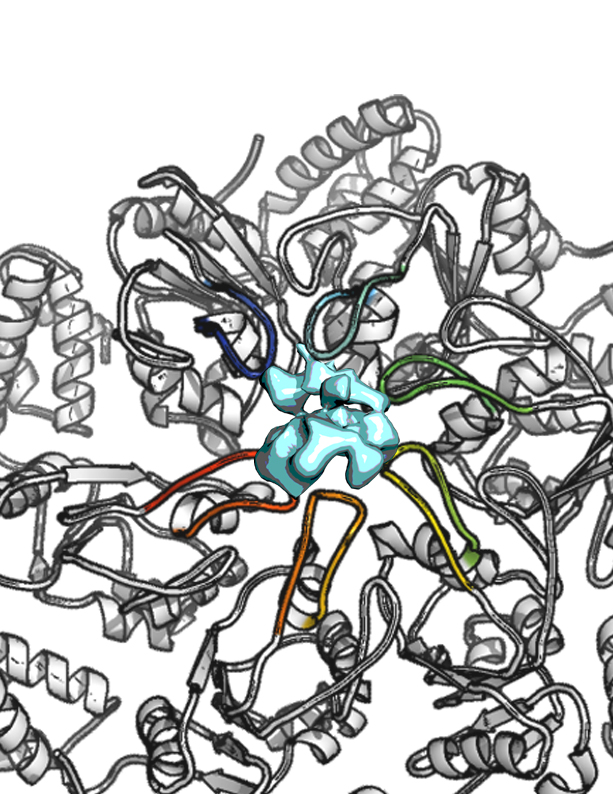
Living creatures use ATP as the “universal energy currency”. ATP-ases are assemblies of molecules that break down ATP into smaller molecules using the energy released to power myriad biological reactions. Molecular motors are ATP-ases that convert this chemical energy into mechanical work on other molecules. The AAA+ ATPases are examples of such molecular machines that perform mechanical work to remodel nearly every type of macromolecule, in cells from all kingdoms of life. A long-standing, largely unanswered question about the functional mechanism of the AAA+ ATPases is how do the rings of chemically identical subunits that make up these assemblies interact with their target macromolecules? The authors address this question by studying Enhancer Binding Proteins (bEBPs) in bacteria, AAA+ ATPases that remodel the σ54-form of RNA polymerase (Eσ54) that is present in complexes with promoter DNA. This remodeling or shape transformation is essential to allow transcription and subsequent expression of genes that allow for nutrient acquisition, complex developmental programs, and virulence as pathogens.
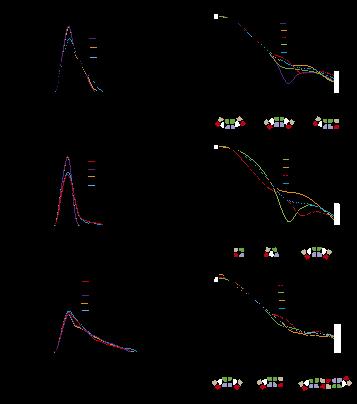
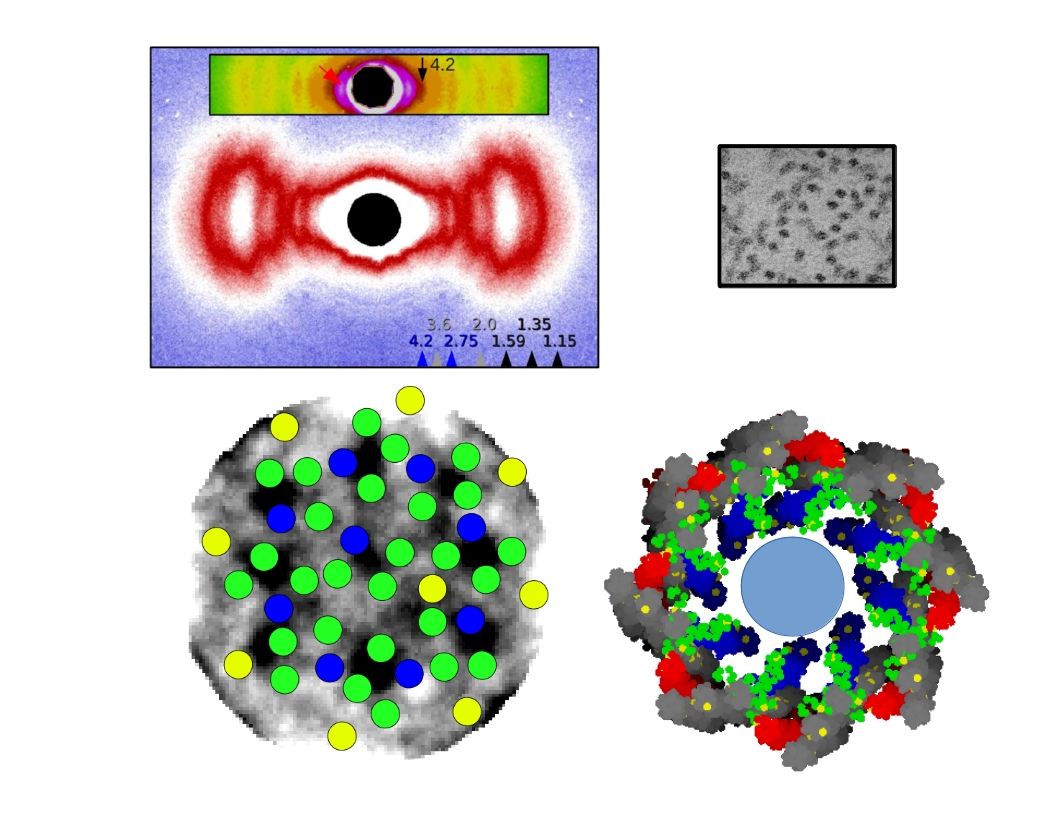
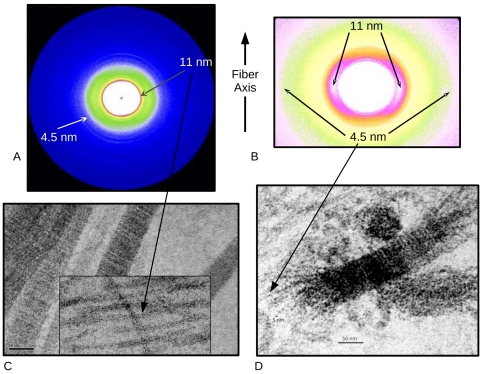
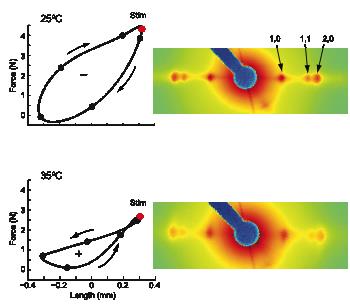
In the current work the authors used isothermal calorimetry (ITC), crystallography and 3D reconstruction from EM single particles along with time-resolved and static small angle X-ray scattering (TR-SAXS and SAXS, respectively) at BioCAT to monitor …