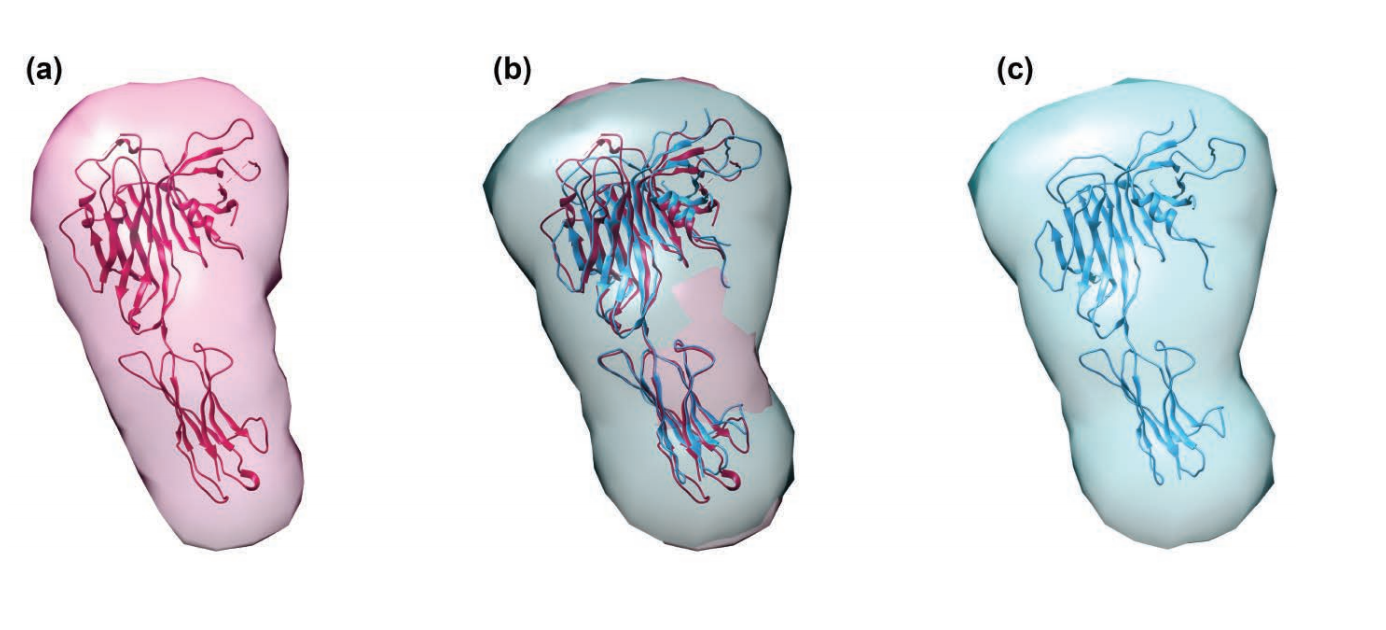
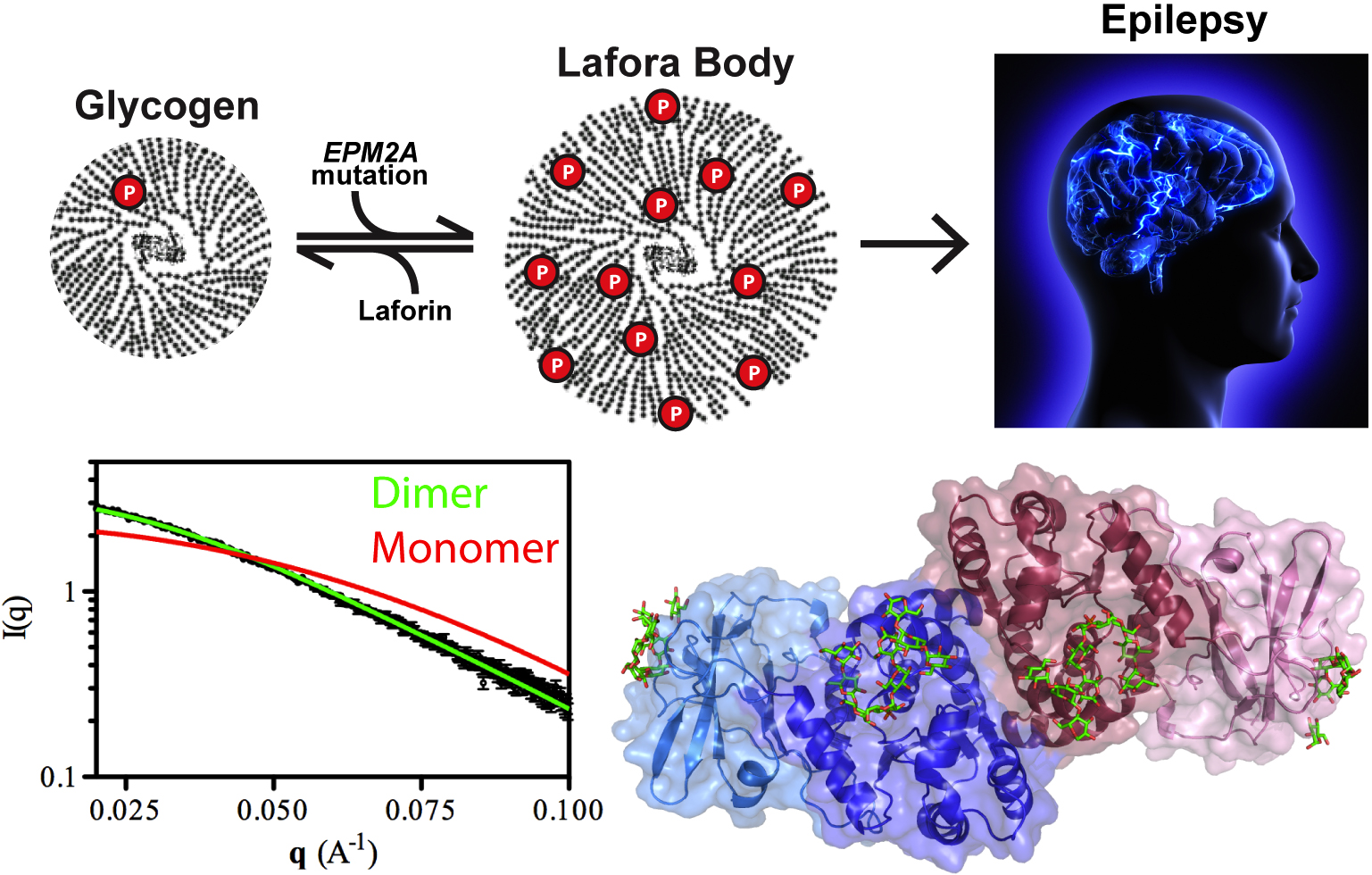
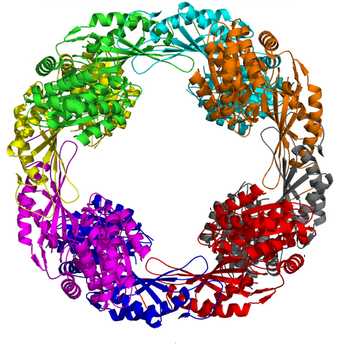
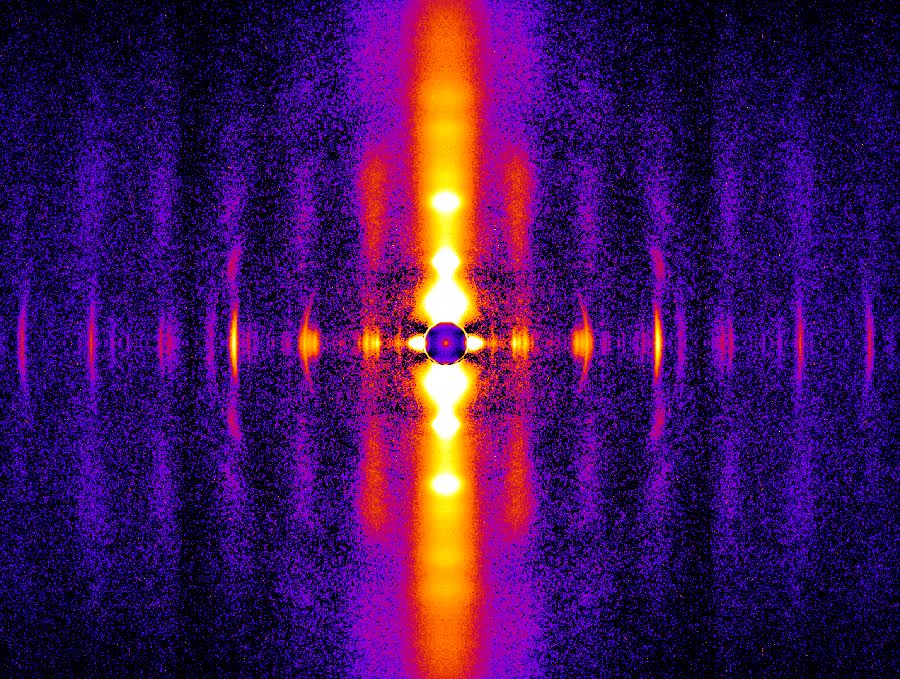
An Unusual Shape Change to Deliver Selenocysteine to Proteins

The element selenium is incorporated into proteins through the 21st amino acid selenocysteine (Sec). Such selenoproteins are critically important to all types of life, suggesting that being able to accurately decode the Sec codon and correctly placing this amino acid in proteins is biologically fundamental. However, little is known about biosynthesis of selenoproteins in eukaryotic cells. To better understand this process, a team of researchers used data gathered at two APS sectors to determine the crystal structure of the human translational elongation factor responsible for recognizing and delivering the transfer rnA (trnA) carrying Sec to the ribosome …
more ...