New study may shed light on protein-drug interactions

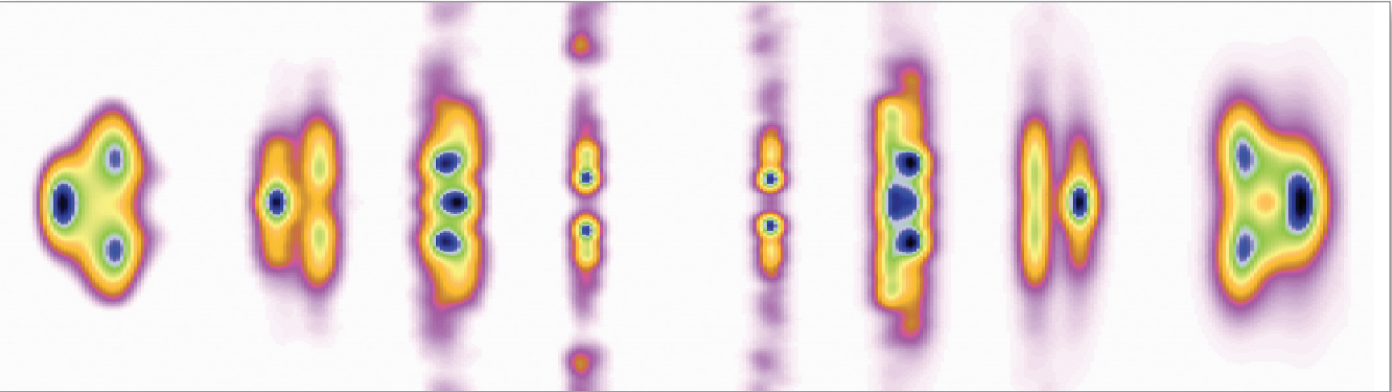
Proteins, the biological molecules that are involved in virtually every action of every organism, may themselves move in surprising ways, according to a recent study from the U.S. Department of Energy’s Argonne National Laboratory that may shed new light on how proteins interact with drugs and other small molecules.
This study, which relied on the intense X-ray beams available at Argonne’s Advanced Photon Source, uses a new approach to characterize the ways in which proteins move around in solution to interact with other molecules, including drugs, metabolites or pieces of DNA.
“Proteins are not static, they’re dynamic,” said Argonne biochemist Lee Makowski, who headed the project. “Part of the common conception of proteins as rigid bodies comes from the fact that we know huge amounts about protein structures but much less about how they move.”
The study of proteins had long focused almost exclusively on their structures, parts of which can resemble chains, sheets or …